Diabetes medication
Drugs used in diabetestreatdiabetes mellitusby decreasingglucose levels in the blood.With the exception ofinsulin,mostGLP-1 receptor agonists(liraglutide,exenatide,and others), andpramlintide,all diabetes medications are administered orally and are thus called oral hypoglycemic agents or oral antihyperglycemic agents. There are different classes of hypoglycemic drugs, and selection of the appropriate agent depends on the nature of diabetes, age, and situation of the person, as well as other patient factors.
Diabetes mellitus type 1is a disease caused by the lack of insulin. Thus, Insulin is the main treatment agent for type 1 and is typically administered via subcutaneous injection.
Diabetes mellitus type 2is a disease of insulin resistance by cells. Type 2 diabetes mellitus is the most common type of diabetes. Treatments include agents that (1) increase the amount of insulin secreted by the pancreas, (2) increase the sensitivity of target organs to insulin, (3) decrease the rate at which glucose is absorbed from the gastrointestinal tract, and (4) increase the loss of glucose through urination.
Several drug classes are indicated for use in type 2 diabetes and are often used in combination. Therapeutic combinations may include severalinsulinisoforms or varying classes of oral antihyperglycemic agents. As of 2020, 23 unique antihyperglycemic drug combinations were approved by theFDA.[1]The first triple combinationof oral anti-diabetics was approved in 2019, consisting ofmetformin,saxagliptin,anddapagliflozin.Another triple combinationapproval formetformin,linagliptin,andempagliflozinfollowed in 2020.[1]
Mechanisms of action
[edit]Diabetes medications have four main mechanisms of action:[citation needed]
- Insulin sensitization:Increased sensitivity of insulin receptors on cells leading to decreasedinsulin resistance,and higher effects of insulin on blood glucose levels.
- Stimulation of beta cells:This stimulation increases insulin secretion frombeta cellsofpancreas.
- Alpha-glucosidase inhibition:Inhibition of thealpha-glucosidaseenzyme, decreases the rate at which glucose is absorbed from the gastrointestinal tract.[2]
- SGLT2 inhibition:Inhibition ofsodium-glucose transport protein 2(SGLT2) decreases glucose reabsorption in the renal tubules of nephrons, thus increasing the amount of glucose excreted in urine.
Insulin
[edit]Insulin is usually givensubcutaneously,either by injections or by aninsulin pump.In acute care settings, insulin may also be given intravenously. Insulins are typically characterized by the rate at which they are metabolized by the body, yielding different peak times and durations of action.[3]Faster-acting insulins peak quickly and are subsequently metabolized, while longer-acting insulins tend to have extended peak times and remain active in the body for more significant periods.[4]
Examples of rapid-acting insulins (peak at ~1 hour) are:[citation needed]
- Insulin lispro(Humalog)
- Insulin aspart(Novolog)
- Insulin glulisine(Apidra)
Examples of short-acting insulins (peak 2–4 hours) are:
- Regular insulin (Humulin R, Novolin R)
- Prompt insulin zinc (Semilente)
Examples of intermediate-acting insulins (peak 4–10 hours) are:
- Isophane insulin, neutral protamine Hagedorn (NPH) (Humulin N, Novolin N)
- Insulin zinc (Lente)
Examples of long-acting insulins (duration 24 hours, often without peak) are:
- Extended insulin zinc insulin (Ultralente)
- Insulin glargine (Lantus)
- Insulin detemir (Levemir)
- Insulin degludec (Tresiba)
Insulin degludec is sometimes classed separately as an "ultra-long" acting insulin due to its duration of action of about 42 hours, compared with 24 hours for most other long-acting insulin preparations.[4]
As a systematic review of studies comparing insulin detemir, insulin glargine, insulin degludec and NPH insulin did not show any clear benefits or seriousadverse effectsfor any particular form of insulin for nocturnalhypoglycemia,severe hypoglycemia,glycated hemoglobinA1c, non-fatalmyocardial infarction/stroke,health-related quality of lifeorall-cause mortality.[5]The same review did not find any differences in effects of using these insulin analogues between adults and children.[5]
Most oral anti-diabetic agents are contraindicated in pregnancy, in which case insulin is preferred.[6]
Insulin is not administered by other routes, although this has been studied. An inhaled form was briefly licensed but was subsequently withdrawn.[7]
Sensitizers
[edit]Insulin sensitizers address the core problem in type 2 diabetes –insulin resistance.
Biguanides
[edit]Biguanidesreducehepaticglucose output and increase uptake of glucose by the periphery, including skeletal muscle. Although it must be used with caution in patients with impaired liver orkidneyfunction,Metformin,a biguanide, has become the most commonly used agent for type 2 diabetes in children and teenagers. Among common diabetic drugs, Metformin is the only widely used oral drug that does not cause weight gain.[8]
Typical reduction inglycated hemoglobin(A1C) values for Metformin is 1.5–2.0%
- Metformin(Glucophage) may be the best choice for patients who also have heart failure,[9]but it should be temporarily discontinued before any radiographic procedure involving intravenousiodinatedcontrast,as patients are at an increased risk oflactic acidosis.
- Phenformin(DBI) was used from 1960s through 1980s, but was withdrawn due to lactic acidosis risk.[10]
- Buforminalso was withdrawn due to lactic acidosis risk.[11]
Metformin is a first-line medication used for treatment of type 2 diabetes. It is generally prescribed at initial diagnosis in conjunction with exercise and weight loss, as opposed to the past, where it was prescribed after diet and exercise had failed. There is an immediate-release as well as an extended-release formulation, typically reserved for patients experiencinggastrointestinalside-effects. It is also available in combination with other oral diabetic medications.
Thiazolidinediones
[edit]Thiazolidinediones(TZDs), also known as "glitazones," bind toPPARγ,peroxisome proliferator activated receptorγ,a type of nuclear regulatory protein involved in the transcription of genes that regulate glucose and fat metabolism. These PPARs act on peroxisome proliferator responsive elements (PPRE).[12]The PPREs influence insulin-sensitive genes, which enhance production of mRNAs of insulin-dependent enzymes. The final result is better use of glucose by the cells. These drugs also enhance PPAR-α activity and hence lead to a rise in HDL and some larger components of LDL.[13]
Typical reductions inglycated hemoglobin(A1C) values are 1.5–2.0%. Some examples are:
- Rosiglitazone(Avandia): theEuropean Medicines Agencyrecommended in September 2010 that it be suspended from the EU market due to elevated cardiovascular risks.[14]
- Pioglitazone(Actos): remains on the market but has also been associated with increased cardiovascular risks.[15]
- Troglitazone(Rezulin): used in 1990s, withdrawn due tohepatitisand liver damage risk.[16]
Multiple retrospective studies have resulted in a concern about rosiglitazone's safety, although it is established that the group, as a whole, has beneficial effects on diabetes. The greatest concern is an increase in the number of severe cardiac events in patients taking it. The ADOPT study showed that initial therapy with drugs of this type may prevent the progression of disease,[17]as did the DREAM trial.[18]TheAmerican Association of Clinical Endocrinologists(AACE), which provides clinical practice guidelines for management of diabetes, retains thiazolidinediones as recommended first, second, or third line agents for type 2 diabetes mellitus, as of their 2019 executive summary, over sulfonylureas and α-glucosidase inhibitors. However, they are less preferred than GLP-1 agonists or SGLT2 inhibitors, especially in patients with cardiovascular disease (whichliraglutide,empagliflozin,andcanagliflozinare all FDA approved to treat).[19]
Concerns about the safety of rosiglitazone arose when a retrospective meta-analysis was published inthe New England Journal of Medicine.[20]There have been a significant number of publications since then, and aFood and Drug Administrationpanel[21]voted, with some controversy, 20:3 that available studies "supported a signal of harm", but voted 22:1 to keep the drug on the market. The meta-analysis was not supported by an interim analysis of the trial designed to evaluate the issue, and several other reports have failed to conclude the controversy. This weak evidence for adverse effects has reduced the use of rosiglitazone, despite its important and sustained effects onglycemic control.[22]Safety studies are continuing.
In contrast, at least one large prospective study, PROactive 05, has shown thatpioglitazonemay decrease the overall incidence of cardiac events in people with type 2 diabetes who have already had a heart attack.[23]
LYN Kinase Activators
[edit]TheLYNkinase activatorTolimidonehas been reported to potentiate insulin signaling in a manner that is distinct from the glitazones.[24]The compound has demonstrated positive results in a Phase 2a clinical study involving 130 diabetic subjects.[25]
Secretagogues
[edit]Secretagoguesare drugs that increase output from a gland, in the case of insulin from thepancreas.
Sulfonylureas
[edit]Sulfonylureaswere the first widely used oral anti-hyperglycemic medications. They areinsulin secretagogues,triggering insulin release by inhibiting theKATPchannel of the pancreaticbeta cells.Eight types of these pills have been marketed in North America, but not all remain available. The "second-generation" sulfonylureas are now more commonly used. They are more effective than first-generation drugs and have fewer side-effects. All may cause weight gain.
Current clinical practice guidelines from theAACErate sulfonylureas (as well as glinides) below all other classes of antidiabetic drugs in terms of suggested use as first, second, or third line agents - this includesBromocriptine,the bile acid sequestrantColesevelam,α-glucosidase inhibitors,Thiazolidinediones(glitazones), andDPP-4 inhibitors(gliptins).[19]The low cost of most sulfonylureas, however, especially when considering their significant efficacy in blood glucose reduction, tends to keep them as a more feasible option in many patients - neither SGLT2 inhibitors nor GLP-1 agonists, the classes most favored by the AACE guidelines after metformin, are currently available as generics.
Sulfonylureas bind strongly toplasma proteins.Sulfonylureas are useful only in type 2 diabetes, as they work by stimulating endogenous release of insulin. They work best with patients over 40 years old who have had diabetes mellitus for under ten years. They cannot be used with type 1 diabetes, or diabetes of pregnancy. They can be safely used with metformin or glitazones. The primary side-effect ishypoglycemia,which appears to happen more commonly with sulfonylureas than with other treatments.[26]
ACochranesystematic reviewfrom 2011 showed that treatment withSulfonylureasdid not improve control of glucose levels more than insulin at 3 nor 12 months of treatment.[27]This same review actually found evidence that treatment with Sulfonylureas could lead to earlier insulin dependence, with 30% of cases requiring insulin at 2 years.[27]When studies measured fastingC-peptide,no intervention influenced its concentration, but insulin maintained concentration better compared to Sulphonylurea.[27]Still, it is important to highlight that the studies available to be included in this review presented considerable flaws in quality and design.[27]
Typical reductions inglycated hemoglobin(A1C) values for second-generation sulfonylureas are 1.0–2.0%.
- First-generation agents
- Second-generation agents
- glipizide
- glyburide orglibenclamide
- glimepiride
- gliclazide
- glyclopyramide
- gliquidone
Meglitinides
[edit]Meglitinideshelp the pancreas produce insulin and are often called "short-acting secretagogues." They act on the same potassium channels as sulfonylureas, but at a different binding site.[28]By closing the potassium channels of the pancreatic beta cells, they open the calcium channels, thereby enhancing insulin secretion.[29]
They are taken with or shortly before meals to boost the insulin response to each meal. If a meal is skipped, the medication is also skipped.
Typical reductions inglycated hemoglobin(A1C) values are 0.5–1.0%.
Adverse reactions include weight gain and hypoglycemia.
Alpha-glucosidase inhibitors
[edit]Alpha-glucosidase inhibitorsare a class of diabetes drugs, however, they are technically not hypoglycemic agents because they do not have a direct effect on insulin secretion or sensitivity. These agents slow the digestion of starch in the small intestine, such that glucose from the starch enters the bloodstream at a slower rate, and can be matched more effectively by an impaired insulin response or sensitivity. These agents are effective by themselves only in the earliest stages ofimpaired glucose tolerance,but can be helpful in combination with other agents intype 2 diabetes.
Typical reductions inglycated hemoglobin(A1C) values are 0.5–1.0%.
These medications are rarely used in the United States because of the severity of their side-effects (flatulence and bloating). They are more commonly prescribed in Europe. They do have the potential to cause weight loss by lowering the amount of sugar metabolized.
Peptide analogs
[edit]This sectionneeds additional citations forverification.(January 2016) |
Injectable incretin mimetics
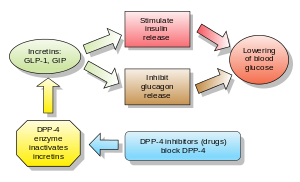
[edit]Incretinsare also insulinsecretagogues.The two main candidate molecules that fulfill criteria for being an incretin areglucagon-like peptide-1(GLP-1) andgastric inhibitory peptide(glucose-dependent insulinotropic peptide, GIP). Both GLP-1 and GIP are rapidly inactivated by the enzymedipeptidyl peptidase-4(DPP-4).
Injectable glucagon-like peptide analogs and agonists
[edit]Glucagon-like peptide (GLP) agonists bind to a membrane GLP receptor.[29]As a consequence, insulin release from the pancreatic beta cells is increased. Endogenous GLP has a half-life of only a few minutes, thus an analogue of GLP would not be practical. As of 2019, theAACElists GLP-1 agonists, along with SGLT2 inhibitors, as the most preferred anti-diabetic agents after metformin.Liraglutidein particular may be considered first-line in diabetic patients with cardiovascular disease, as it has received FDA approval for reduction of risk of major adverse cardiovascular events in patients with type 2 diabetes.[19][30]In a 2011Cochranereview,GLP-1 agonists showed approximately a 1% reduction in HbA1c when compared to placebo.[26]GLP-1 agonists also show improvement ofbeta-cellfunction, but this effect does not last after treatment is stopped.[26]Due to shorter duration of studies, this review did not allow for long-term positive or negative effects to be assessed.[26]
- Exenatide(also Exendin-4, marketed as Byetta) is the firstGLP-1agonist approved for the treatment oftype 2 diabetes.Exenatide is not an analogue of GLP but rather a GLP agonist.[31][32]Exenatide has only 53% homology with GLP, which increases its resistance to degradation by DPP-4 and extends its half-life.[33]A 2011 Cochrane review showed a HbA1c reduction of 0.20% more with Exenatide 2 mg compared to insulin glargine, exenatide 10 μg twice daily, sitagliptin and pioglitazone.[26]Exenatide, together with liraglutide, led to greater weight loss than glucagon-like peptide analogues.[26]
- Liraglutide,a once-daily human analogue (97% homology), has been developed byNovo Nordiskunder the brand nameVictoza.The product was approved by theEuropean Medicines Agency(EMEA) on July 3, 2009, and by theU.S. Food and Drug Administration(FDA) on January 25, 2010.[34][35][36][37][38][39]A 2011 Cochrane review showed a HbA1c reduction of 0.24% more with liraglutide 1.8 mg compared to insulin glargine, 0.33% more than exenatide 10 μg twice daily, sitagliptin and rosiglitazone.[26]Liraglutide, together with exenatide, led to greater weight loss than glucagon-like peptide analogues.[26]
- Taspoglutideis presently in Phase III clinical trials withHoffman-La Roche.
- Lixisenatide (Lyxumia) Sanofi Aventis
- Semaglutide(Ozempic) (oral version is Rybelsus)
- Dulaglutide(Trulicity) - once weekly
- Albiglutide(Tanzeum) - once weekly
- Tirzepatide(dual GLP-1 andGIPagonist; manufactured byEli Lilly,and approved in 2022. It is Marketed under brandname Mounjaro for type II diabetes, and Zepbound for obesity[40]
These agents may also cause a decrease in gastric motility, responsible for the common side-effect of nausea, which tends to subside with time.[26]
Gastric inhibitory peptide analogs
[edit]Dipeptidyl peptidase-4 inhibitors
[edit]GLP-1 analogs resulted in weight loss and had more gastrointestinal side-effects, while in generaldipeptidyl peptidase-4(DPP-4) inhibitors were weight-neutral and are associated with increased risk for infection and headache. Both classes appear to present an alternative to other antidiabetic drugs. However, weight gain and/or hypoglycemia have been observed whendipeptidyl peptidase-4 inhibitorswere used with sulfonylureas; effects on long-term health and morbidity rates are still unknown.[41]
DPP-4 inhibitors increase blood concentration of theincretinGLP-1 by inhibiting its degradation by DPP-4.
Examples are:
- vildagliptin(Galvus) EU Approved 2008
- sitagliptin(Januvia) FDA approved Oct 2006
- saxagliptin(Onglyza) FDA Approved July 2009
- linagliptin(Tradjenta) FDA Approved May 2, 2011
- alogliptin
- septagliptin
- teneligliptin
- gemigliptin(Zemiglo)
DPP-4 inhibitors lowered hemoglobinA1Cvalues by 0.74%, comparable to other antidiabetic drugs.[42]
A result in one RCT comprising 206 patients aged 65 or older (mean baseline HgbA1c of 7.8%) receiving either 50 or 100 mg/d ofsitagliptinwas shown to reduce HbA1c by 0.7% (combined result of both doses).[43]A combined result of 5 RCTs enlisting a total of 279 patients aged 65 or older (mean baseline HbA1c of 8%) receiving 5 mg/d ofsaxagliptinwas shown to reduce HbA1c by 0.73%.[44]A combined result of 5 RCTs enlisting a total of 238 patients aged 65 or older (mean baseline HbA1c of 8.6%) receiving 100 mg/d ofvildagliptinwas shown to reduce HbA1c by 1.2%.[45]Another set of 6 combined RCTs involvingalogliptin(approved by FDA in 2013) was shown to reduce HbA1c by 0.73% in 455 patients aged 65 or older who received 12.5 or 25 mg/d of the medication.[46]
Injectable amylin analogues
[edit]Amylinagonist analogues slow gastric emptying and suppressglucagon.They have all the incretins actions except stimulation of insulin secretion. As of 2007[update],pramlintideis the only clinically available amylin analogue. Like insulin, it is administered bysubcutaneous injection.The most frequent and severe adverse effect of pramlintide isnausea,which occurs mostly at the beginning of treatment and gradually reduces. Typical reductions in A1C values are 0.5–1.0%.[47]
SGLT2 inhibitors
[edit]SGLT2 inhibitorsblock the sodium-glucose linked transporter 2 proteins inrenal tubulesofnephronsin kidneys, reabsorption of glucose in into the renal tubules, promoting excretion of glucose in the urine. This causes both mild weight loss, and a mild reduction in blood sugar levels with little risk of hypoglycemia.[48]Oral preparations may be available alone or in combination with other agents.[49]Along with GLP-1 agonists, they are considered preferred second or third agents for type 2 diabetics sub-optimally controlled with metformin alone, according to most recent clinical practice guidelines.[19]Because they are taken by mouth, rather than injected (like GLP-1 agonists), patients who areinjection-aversemay prefer these agents over the former. They may be considered first line in diabetic patients with cardiovascular disease, especiallyheart failure,as these medications have been shown to reduce the risk of hospitalization in patients with such comorbidities.[50]Because they are not available as generic medications, however, cost may limit their feasibility for many patients. Furthermore, there has been growing evidence that the effectiveness and safety of this drug class could depend on genetic variability of the patients.[51]
Examples include:
The side effects of SGLT2 inhibitors are derived directly from their mechanism of action; these include an increased risk of:ketoacidosis,urinary tract infections,candidal vulvovaginitis,andhypoglycemia.[52]
Comparison
[edit]The following table compares some common anti-diabetic agents, generalizing classes, although there may be substantial variation in individual drugs of each class. When the table makes a comparison such as "lower risk" or "more convenient" the comparison is with the other drugs on the table.
| Comparison of anti-diabetic medication[53][54] | ||||
|---|---|---|---|---|
| Drug class[54] | Mechanism of action[6] | Advantages[54] | Disadvantages[54] | |
| Sulfonylureas(glyburide,glimepiride,glipizide) | Stimulating insulin release by pancreaticbeta cellsby inhibiting theKATPchannel |
|
| |
| Metformin | Acts on the liver to reduce gluconeogenesis and causes a decrease ininsulin resistancevia increasingAMPKsignalling. |
|
| |
| Alpha-glucosidase inhibitors(acarbose,miglitol,voglibose) | Inhibit carbohydrate digestion in the small intestine by inhibiting enzymes that break down polysaccharides |
|
| |
| Thiazolidinediones(Pioglitazone,Rosiglitazone) | Reduce insulin resistance by activatingPPAR-γin fat and muscle |
|
| |
| SGLT2 inhibitors | ||||
Generics
[edit]Many anti-diabetes drugs are available as generics. These include:[55]
- Sulfonylureas– glimepiride, glipizide, glyburide
- Biguanides– metformin
- Thiazolidinediones(Tzd) – pioglitazone, Actos generic
- Alpha-glucosidase inhibitors– Acarbose
- Meglitinides– nateglinide
- Combination of sulfonylureas plus metformin – known by generic names of the two drugs
No generics are available fordipeptidyl peptidase-4 inhibitors(Onglyza), the glifozins, the incretins and various combinations. Sitagliptin patent expired in July 2022, leading to launch of generic sitagliptin[56]brands. This lowered the cost of therapy for type 2 diabetes using sitagliptin.
Alternative Medicine
[edit]The effect ofAyurvedictreatments has been researched, however due to methodological flaws of relevant studies and research, it has not been possible to draw conclusions regarding efficacy of these treatments and there is insufficient evidence to recommend them.[57]
References
[edit]- ^abDahlén AD, Dashi G, Maslov I, Attwood MM, Jonsson J, Trukhan V, et al. (January 2022)."Trends in Antidiabetic Drug Discovery: FDA Approved Drugs, New Drugs in Clinical Trials and Global Sales".Front Pharmacol.12:4119.doi:10.3389/fphar.2021.807548.PMC8807560.PMID35126141.
- ^"OVERVIEW OF DIABETES DRUGS".diabetes daily.
- ^Powers AC (2011). "Diabetes Mellitus". In Longo DL,Fauci AS,Kasper DL, Hauser SL, Jameson JL, Loscalzo J (eds.).Harrison's Principles of Internal Medicine(18th ed.). McGraw-Hill.ISBN978-0071748896.
- ^abDonner T, Sarkar S (2000)."Insulin – Pharmacology, Therapeutic Regimens, and Principles of Intensive Insulin Therapy".In Feingold KR, Anawalt B, Boyce A, Chrousos G (eds.).Endotext.MDText.com, Inc.PMID25905175.RetrievedNovember 16,2019.
- ^abHemmingsen B, Metzendorf MI, Richter B (March 2021)."(Ultra-)long-acting insulin analogues for people with type 1 diabetes mellitus".The Cochrane Database of Systematic Reviews.3(4): CD013498.doi:10.1002/14651858.cd013498.pub2.PMC8094220.PMID33662147.
- ^abcdTable entries taken from page 185 in:Elizabeth D Agabegi, Agabegi, Steven S. (2008).Step-Up to Medicine (Step-Up Series).Hagerstwon, MD: Lippincott Williams & Wilkins.ISBN978-0-7817-7153-5.
- ^Mastrandrea LD (March 2010)."Inhaled insulin: overview of a novel route of insulin administration".Vascular Health and Risk Management.6:47–58.doi:10.2147/VHRM.S6098.PMID20234779.
- ^"Erratum: Metformin: Current knowledge".Journal of Research in Medical Sciences.29(1): 6. January 4, 2024.doi:10.4103/JRMS.JRMS_62_24.ISSN1735-1995.PMC10956562.PMID38524744.
- ^Eurich DT, McAlister FA, Blackburn DF, Majumdar SR, Tsuyuki RT, Varney J, et al. (September 2007)."Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review".BMJ.335(7618): 497.doi:10.1136/bmj.39314.620174.80.PMC1971204.PMID17761999.
- ^Fimognari FL, Pastorelli R, Incalzi RA (April 2006)."Phenformin-induced lactic acidosis in an older diabetic patient: a recurrent drama (phenformin and lactic acidosis)".Diabetes Care.29(4): 950–951.doi:10.2337/diacare.29.04.06.dc06-0012.PMID16567854.Archivedfrom the original on December 9, 2012.
- ^Verdonck LF, Sangster B, van Heijst AN, de Groot G, Maes RA (1981)."Buformin concentrations in a case of fatal lactic acidosis".Diabetologia.20(1): 45–46.doi:10.1007/BF01789112.PMID7202882.
- ^"diabetesinsulinPPAR".www.healthvalue.net.Archivedfrom the original on March 3, 2016.RetrievedMay 6,2018.
- ^Kersten S (January 2, 2008). Chinetti G (ed.)."Peroxisome proliferator activated receptors and lipoprotein metabolism".PPAR Research.2008(1): 132960.doi:10.1155/2008/132960.PMID18288277.
- ^European Medicines Agency,"European Medicines Agency recommends suspension of Avandia, Avandamet and Avaglim"ArchivedFebruary 3, 2014, at theWayback Machine,EMA,23 September 2009
- ^Lincoff AM, Wolski K, Nicholls SJ, Nissen SE (September 2007). "Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials".JAMA.298(10): 1180–1188.doi:10.1001/jama.298.10.1180.PMID17848652.
- ^Hinterthuer A (October 1, 2008)."Retired Drugs: Failed Blockbusters, Homicidal Tampering, Fatal Oversights".Wired News.Archivedfrom the original on December 4, 2008.RetrievedJune 21,2009.
- ^Haffner SM (2007)."Expert Column – A Diabetes Outcome Progression Trial (ADOPT)".Medscape.RetrievedSeptember 21,2007.
- ^Gagnon L (October 24, 2006)."DREAM: Rosiglitazone Effective in Preventing Diabetes".Medscape.Archivedfrom the original on December 2, 2008.RetrievedSeptember 21,2007.
- ^abcdGarber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. (January 2019)."Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2019 Executive Summary".Endocrine Practice.25(1): 69–100.doi:10.4158/cs-2018-0535.PMID30742570.
- ^Nissen SE, Wolski K (June 2007)."Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes".The New England Journal of Medicine.356(24): 2457–2471.doi:10.1056/NEJMoa072761.PMID17517853.
- ^Wood S (July 31, 2007)."FDA Advisory Panels Acknowledge Signal of Risk With Rosiglitazone, but Stop Short of Recommending Its Withdrawal".Heartwire.Archivedfrom the original on March 18, 2014.RetrievedSeptember 21,2007.
- ^Ajjan RA, Grant PJ (July 2008). "The cardiovascular safety of rosiglitazone".Expert Opinion on Drug Safety.7(4): 367–376.doi:10.1517/14740338.7.4.367.PMID18613801.S2CID73109231.
- ^Erdmann E, Dormandy JA, Charbonnel B, Massi-Benedetti M, Moules IK, Skene AM (May 2007)."The effect of pioglitazone on recurrent myocardial infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction: results from the PROactive (PROactive 05) Study".Journal of the American College of Cardiology.49(17): 1772–1780.doi:10.1016/j.jacc.2006.12.048.PMID17466227.
- ^Müller G, Wied S, Frick W (July 2000)."Cross talk of pp125(FAK) and pp59(Lyn) non-receptor tyrosine kinases to insulin-mimetic signaling in adipocytes".Molecular and Cellular Biology.20(13): 4708–4723.doi:10.1128/mcb.20.13.4708-4723.2000.PMC85892.PMID10848597.
- ^"Melior Pharmaceuticals Announces Positive Phase 2A Results in Type 2 Diabetes Study".businesswire.com.June 13, 2016.Archivedfrom the original on August 12, 2017.RetrievedMay 6,2018.
- ^abcdefghiShyangdan DS, Royle P, Clar C, Sharma P, Waugh N, Snaith A (October 2011)."Glucagon-like peptide analogues for type 2 diabetes mellitus".The Cochrane Database of Systematic Reviews.2011(10): CD006423.doi:10.1002/14651858.cd006423.pub2.PMC6486297.PMID21975753.
- ^abcdBrophy S, Davies H, Mannan S, Brunt H, Williams R (September 2011)."Interventions for latent autoimmune diabetes (LADA) in adults".The Cochrane Database of Systematic Reviews.2011(9): CD006165.doi:10.1002/14651858.cd006165.pub3.PMC6486159.PMID21901702.
- ^Rendell M (September 2004)."Advances in diabetes for the millennium: drug therapy of type 2 diabetes".MedGenMed.6(3 Suppl): 9.PMC1474831.PMID15647714.
- ^ab"Helping the pancreas produce insulin".HealthValue.Archivedfrom the original on September 27, 2007.RetrievedSeptember 21,2007.
- ^"Victoza (liraglutide) is Approved to Reduce the Risk of Three Major Adverse Cardiovascular Events in Type 2 Diabetes Patients".Drugs.com.RetrievedNovember 16,2019.
- ^Briones M, Bajaj M (June 2006). "Exenatide: a GLP-1 receptor agonist as novel therapy for Type 2 diabetes mellitus".Expert Opinion on Pharmacotherapy.7(8): 1055–1064.doi:10.1517/14656566.7.8.1055.PMID16722815.S2CID43740629.
- ^Gallwitz B (December 2006)."Exenatide in type 2 diabetes: treatment effects in clinical studies and animal study data".International Journal of Clinical Practice.60(12): 1654–1661.doi:10.1111/j.1742-1241.2006.01196.x.PMID17109672.S2CID8800490.
- ^Cvetković RS, Plosker GL (2007). "Exenatide: a review of its use in patients with type 2 diabetes mellitus (as an adjunct to metformin and/or a sulfonylurea)".Drugs.67(6): 935–954.doi:10.2165/00003495-200767060-00008.PMID17428109.S2CID195691202.
- ^"Novo Nordisk Files for Regulatory Approval of Liraglutide in Both the US and Europe".Archivedfrom the original on December 15, 2017.RetrievedJanuary 23,2018.May 2008
- ^"Liraglutide Provides Significantly Better Glucose Control Than Insulin Glargine in Phase 3 Study".Archivedfrom the original on July 23, 2010.RetrievedFebruary 9,2010."Liraglutide Provides Significantly Better Glucose Control Than Insulin Glargine In Phase 3 Study" June 2007
- ^"Clinical Study Shows Liraglutide Reduced Blood Sugar, Weight, and Blood Pressure in Patients with Type 2 Diabetes".Archivedfrom the original on February 5, 2009.RetrievedFebruary 9,2010."Clinical Study Shows Liraglutide Reduced Blood Sugar, Weight, And Blood Pressure In Patients With Type 2 Diabetes" June 2008
- ^"Liraglutide – Next-Generation Antidiabetic Medication".Archivedfrom the original on June 18, 2010.RetrievedFebruary 9,2010.
- ^"Quarterly R&D; Update - Novo Nordisk A/S".Archived fromthe originalon January 9, 2010.RetrievedFebruary 9,2010.Oct 2008 Inc results of LEAD 6 extension
- ^"Novo Nordisk Receives US Approval for Victoza(R) (Liraglutide) for the Treatment of Type 2 Diabetes".Archivedfrom the original on January 29, 2010.RetrievedFebruary 9,2010.January 2009
- ^Frías JP,Davies MJ,Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. (August 2021)."Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes".The New England Journal of Medicine.385(6): 503–515.doi:10.1056/NEJMoa2107519.PMID34170647.S2CID235635529.
{{cite journal}}:CS1 maint: overridden setting (link) - ^National Prescribing Service (August 1, 2010)."Dipeptidyl peptidase-4 inhibitors ('gliptins') for type 2 diabetes mellitus".RADAR.RetrievedMarch 7,2021.
- ^Amori RE, Lau J, Pittas AG (July 2007). "Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis".JAMA.298(2): 194–206.doi:10.1001/jama.298.2.194.PMID17622601.
- ^Barzilei N, Mahoney EM, Guo H (2009). "Sitagliptin is well tolerated and leads to rapid improvement in blood glucose in the first days of monotherapy in patients aged 65 years and older with T2DM".Diabetes.58:587.
- ^Doucet J, Chacra A, Maheux P, Lu J, Harris S, Rosenstock J (April 2011). "Efficacy and safety of saxagliptin in older patients with type 2 diabetes mellitus".Current Medical Research and Opinion.27(4): 863–869.doi:10.1185/03007995.2011.554532.PMID21323504.S2CID206965817.
- ^Pratley RE, Rosenstock J, Pi-Sunyer FX, Banerji MA, Schweizer A, Couturier A, et al. (December 2007)."Management of type 2 diabetes in treatment-naive elderly patients: benefits and risks of vildagliptin monotherapy".Diabetes Care.30(12): 3017–3022.doi:10.2337/dc07-1188.PMID17878242.
- ^Pratley RE, McCall T, Fleck PR, Wilson CA, Mekki Q (November 2009). "Alogliptin use in elderly people: a pooled analysis from phase 2 and 3 studies".Journal of the American Geriatrics Society.57(11): 2011–2019.doi:10.1111/j.1532-5415.2009.02484.x.PMID19793357.S2CID28683917.
- ^Ryan G, Briscoe TA, Jobe L (February 2009)."Review of pramlintide as adjunctive therapy in treatment of type 1 and type 2 diabetes".Drug Design, Development and Therapy.2:203–214.doi:10.2147/DDDT.S3225.PMID19920907.
- ^Dietrich E, Powell J, Taylor JR (November 2013)."Canagliflozin: a novel treatment option for type 2 diabetes".Drug Design, Development and Therapy.7:1399–1408.doi:10.2147/DDDT.S48937.PMC3840773.PMID24285921.
- ^Center for Drug Evaluation and Research."Drug Safety and Availability - Sodium-glucose Cotransporter-2 (SGLT2) Inhibitors".www.fda.gov.Archivedfrom the original on November 29, 2016.RetrievedAugust 26,2017.
- ^"UpToDate".www.uptodate.com.RetrievedNovember 16,2019.
- ^Imamovic Kadric S, Kulo Cesic A, Dujic T. Pharmacogenetics of new classes of antidiabetic drugs. Bosn J of Basic Med Sci. 2021.DOI:https://doi.org/10.17305/bjbms.2021.5646
- ^"SGLT2 Inhibitors (Gliflozins) – Drugs, Suitability, Benefits & Side Effects".Archivedfrom the original on August 27, 2017.RetrievedAugust 26,2017.
- ^Cambon-Thomsen A, Rial-Sebbag E, Knoppers BM (August 2007)."Trends in ethical and legal frameworks for the use of human biobanks".The European Respiratory Journal.30(2): 373–382.doi:10.1183/09031936.00165006.PMID17666560.adapted from table 2, which includes a list of issues
- ^abcdConsumer Reports Health Best Buy Drugs."The Oral Diabetes Drugs: Treating Type 2 Diabetes"(PDF).Best Buy Drugs.Consumer Reports:20.Archived(PDF)from the original on February 27, 2013.RetrievedSeptember 18,2012.,which is citing
- Agency for Healthcare Research and Quality(March 2011)."Oral Diabetes Medications for Adults With Type 2 Diabetes. An Update"(PDF).Comparative Effectiveness Review(27). Archived fromthe original(PDF)on September 27, 2013.RetrievedNovember 28,2012.
- Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, et al. (May 2011)."Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations".Annals of Internal Medicine.154(9): 602–613.doi:10.7326/0003-4819-154-9-201105030-00336.PMC3733115.PMID21403054.
- ^"The Oral Diabetes Drugs Treating Type 2 Diabetes Comparing Effectiveness, Safety, and Price"(PDF).Archived(PDF)from the original on June 15, 2013.RetrievedJuly 17,2013.
- ^"Sitagliptin Generic Alternatives".www.sastimedic.com.January 31, 2024.RetrievedJanuary 31,2024.
- ^Sridharan K, Mohan R, Ramaratnam S, Panneerselvam D, et al. (Cochrane Metabolic and Endocrine Disorders Group) (December 2011)."Ayurvedic treatments for diabetes mellitus".The Cochrane Database of Systematic Reviews(12): CD008288.doi:10.1002/14651858.CD008288.pub2.PMC3718571.PMID22161426.
Further reading
[edit]- Lebovitz, Harold E. (2004).Therapy For Diabetes Mellitus and Related Disorders(4th ed.). Alexandria, VA:American Diabetes Association.ISBN978-1-58040-187-6.
- Adams, Michael Ian, Holland, Norman Norwood (2003).Core Concepts in Pharmacology.Englewood Cliffs, NJ: Prentice Hall.ISBN978-0-13-089329-1.
