Bat virome

Thebat viromeis thegroup of virusesassociated withbats.Bats host a diverse array of viruses, including all seven types described by theBaltimore classification system:(I)double-stranded DNA viruses;(II)single-stranded DNA viruses;(III)double-stranded RNA viruses;(IV)positive-sense single-stranded RNA viruses;(V)negative-sense single-stranded RNA viruses;(VI)positive-sense single-stranded RNA viruses that replicate through a DNA intermediate;and (VII)double-stranded DNA viruses that replicate through a single-stranded RNA intermediate.The greatest share of bat-associated viruses identified as of 2020 are of type IV, in the familyCoronaviridae.
Bats harbor several viruses that arezoonotic,or capable of infecting humans, and some bat-borne viruses are considered importantemerging viruses.[1][2]These zoonotic viruses include therabies virus,SARS-CoV,MERS-CoV,Marburg virus,Nipah virus,andHendra virus.While research clearly indicates thatSARS-CoV-2originated in bats,[3]it is unknown how it was transmitted to humans, or if an intermediate host was involved. It has been speculated that bats may have a role in theecologyof the Ebola virus, though this is unconfirmed. While transmission of rabies from bats to humans usually occurs via biting, most other zoonotic bat viruses are transmitted by direct contact with infected bat fluids like urine,guano,or saliva, or through contact with an infected, non-batintermediate host.There is no firm evidence that butchering or consumingbat meatcan lead to viral transmission, though this has been speculated.
Despite the abundance of viruses associated with bats, they rarely become ill from viral infections, andrabiesis the only viral illness known to kill bats. Much research has been conducted on batvirology,particularly batimmune response.Bats'immune systemsdiffer from other mammals in their lack of severalinflammasomes,which activate the body's inflammatory response, as well as a dampenedstimulator of interferon genes(STING) response, which helps control host response to pathogens. Preliminary evidence indicates bats are thus more tolerant of infection than other mammals. While much research has centered on bats as a source of zoonotic disease, reviews have found mixed results on whether bats harbor more zoonotic viruses than other groups. A 2015 review found that bats do not harbor more zoonotic viruses thanprimatesorrodents,though the three groups harbored more than other mammalorders.[4]In contrast, a 2020 review found that bats do not have more zoonotic viruses than any other bird or mammal group when viral diversity is measured relative to host diversity, as bats are the second-most diverse order of mammals.[5]
Viral diversity
[edit]| Virus family | No. sequences (n= 10,845) |
|---|---|
| Coronaviridae | |
| Rhabdoviridae | |
| Paramyxoviridae | |
| Astroviridae | |
| Adenoviridae | |
| Polyomaviridae | |
| Reoviridae | |
| Circoviridae | |
| Herpesviridae | |
| Flaviviridae | |
| Picornaviridae | |
| Parvoviridae | |
| Filoviridae | |
| Hepadnaviridae | |
| Papillomaviridae | |
| Hantaviridae | |
| Caliciviridae | |
| Peribunyaviridae | |
| Nairoviridae | |
| Retroviridae | |
| Orthomyxoviridae | |
| Phenuiviridae | |
| Poxviridae | |
| Picobirnaviridae | |
| Togaviridae | |
| Genomoviridae | |
| Bornaviridae | |
| Anelloviridae |
Viruses have been found in bat populations around the world. Bats harbor all groups of viruses in theBaltimore classification,[7]representing at least 28 families of viruses.[6]Most of the viruses harbored by bats areRNA viruses,though they are also known to haveDNA viruses.[8]Bats are more tolerant of viruses than terrestrial mammals.[8]A single bat can host several different kinds of viruses without becoming ill.[9]Bats have also been shown to be more susceptible to reinfection with the same viruses, whereas other mammals, especially humans, have a greater propensity for developing varying degrees of immunity.[10][11]Their behavior and life history also make them "exquisitely suitable hosts of viruses and other disease agents", with long lifespans, the ability to entertorporor hibernate, and their ability to traverse landscapes with daily and seasonal movement.[1]
Though bats harbor diverse viruses, they are rarely lethal to the bat host. Only the rabies virus and a few other lyssaviruses have been confirmed to kill bats.[7]Various factors have been implicated in bats' ability to survive viral infections. One possibility is bats' use of flight. Flight produces afever-like response, resulting in elevated temperature (up to 38 °C (100 °F)) and metabolic rate. Additionally, this fever-like response may help them cope with actual fevers upon getting a viral infection.[7]Some research indicates that bats' immune systems have allowed them to cope with a variety of viruses. A 2018 study found that bats have a dampenedSTING responsecompared to other mammals, which could allow them to respond to viral threats without over-responding.[8]STING is asignaling moleculethat helps coordinate various host defense genes against pathogens.[12]The authors of the study concluded that "the weakened, but not entirely lost, functionality of STING may have profound impact for bats to maintain the balanced state of 'effective response' but not 'over response' against viruses."[8]
Additionally, bats lack severalinflammasomesfound in other mammals;[8]other inflammasomes are present with a greatly reduced response.[13]While inflammation is an immune response to viruses, excessive inflammation is damaging to the body, and viruses likesevere acute respiratory syndrome coronavirus(SARS-CoV) are known to kill humans by inducing excessive inflammation. Bats' immune systems may have evolved to be more tolerant of stressors such as viral infections compared to other mammals.[14]
Transmission to humans
[edit]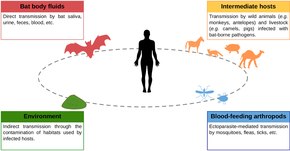
The vast majority of bat viruses have nozoonoticpotential, meaning they cannot be transmitted to humans.[6]The zoonotic viruses have four possible routes of transmission to humans: contact with bat body fluids (blood, saliva, urine, feces); intermediate hosts; environmental exposure; and blood-feeding arthropods.[15]Lyssaviruseslike therabies virusare transmitted from bats to humans via biting. Transmission of most other viruses does not appear to take place via biting, however. Contact with bat fluids such as guano, urine, and saliva is an important source ofspilloverfrom bats to humans. Other mammals may play a role in transmitting bat viruses to people, withpig farmsa source of bat-borne viruses in Malaysia and Australia.[15][16]Other possible transmission routes of bat-borne viruses are more speculative. It is possible but unconfirmed that hunting, butchering, and consuming bat meat can result in viral spillover. Whilearthropodslikemosquitoes,ticks,andfleasmaytransmit viral infectionsfrom other mammals to humans, it is highly speculative that arthropods play a role in mediating bat viruses to humans. There is little evidence of environmental transmission of viruses from bats to humans, meaning that bat-borne virus do not persist in the environment for long. However, a limited number of studies have been conducted on the subject.[15]
Bats compared to other viral reservoirs
[edit]Bats and their viruses may be the subject of more research than viruses found in other mammalorders,an example of research bias. A 2015 review found that from 1999 to 2013, there were 300–1200 papers published about bat viruses annually, compared to 12–45 publications formarsupialviruses and only 1–9 studies forslothviruses. The same review found that bats do not have significantly greater viral diversity than other mammal groups. Bats, rodents, and primates all harbored significantly more zoonotic viruses than other mammal groups, though the differences among the aforementioned three groups were not significant (bats have no more zoonotic viruses than rodents and primates).[4]A 2020 review of mammals and birds found that the identity of the taxonomic groups did not have any impact on the probability of harboring zoonotic viruses. Instead, more diverse groups had greater viral diversity. Bat life history traits and immunity, while likely influential in determining bat viral communities, were not associated with a greater probability of viral spillover into humans.[5]
Sampling
[edit]
Bats are sampled for viruses in a variety of ways. They can be tested for seropositivity for a given virus using a method likeELISA,which determines whether or not they have the correspondingantibodiesfor the virus. They can also be surveyed using molecular detection techniques likePCR(polymerase chain reaction), which can be used to replicate and amplify viral sequences.Histopathology,which is the microscopic examination of tissue, can also be used. Viruses have been isolated from bat blood, saliva, feces, tissue, and urine. Some sampling is non-invasive and does not require killing the bat for sampling, whereas other sampling requires sacrificing the animal first. A 2016 review found no significant difference in total number of viruses found and new viruses discovered between lethal and non-lethal studies. Several species ofthreatenedbat have been killed for viral sampling, including theComoro rousette,Hildegarde's tomb bat,Natal free-tailed bat,and thelong-fingered bat.[17]
Double-stranded DNA viruses
[edit]Adenoviruses
[edit]Adenoviruseshave been detected in bat guano, urine, and oral and rectal swabs. They have been found in bothmegabatsandmicrobatsacross a large geographic area. Bat adenoviruses are closely related to those finds incanids.[18]The greatest diversity of bat adenoviruses has been found in Eurasia, though the virus family may be undersampled in bats overall.[7]
Herpesviruses
[edit]Diverseherpesviruseshave been found in bats in North and South America, Asia, Africa, and Europe,[18]including representatives of the three subfamilies,alpha-,beta-,andgammaherpesviruses.[7]Bat-hosted herpesviruses include the speciesPteropodid alphaherpesvirus 1andVespertilionid gammaherpesvirus 1.[19]
Papillomaviruses
[edit]Papillomaviruseswere first detected in bats in 2006, in theEgyptian fruit bat.They have since been identified in several other bat species, including theserotine bat,greater horseshoe bat,and thestraw-colored fruit bat.Five distinct lineages of bat papillomaviruses have been recognized.[18]
Single-stranded DNA viruses
[edit]Anelloviruses
[edit]Noanellovirusis known to cause disease in humans.[7]The first bat anellovirus, aTorque teno virus,was found in a Mexican free-tailed bat.[20]Novel anelloviruses have also been detected in twoleaf-nosed batspecies: thecommon vampire batandSeba's short-tailed bat.The bat anelloviruses and one opossum anellovirus have been included in the proposed genusSigmatorquevirus.[21]
Circoviruses
[edit]Circoviruses,familyCircoviridae,are among the most diverse of all viruses.[22]Like anelloviruses, circoviruses are not associated with any disease in humans.[7]About a third of all circoviruses are associated with bats, found in North and South America, Europe, and Asia.[22]A study ofhorseshoeandvesperbats in China identified circoviruses from the generaCircovirusandCyclovirus.[23]
Parvoviruses
[edit]Several kinds ofparvovirusesare considered important for human and animal health. Several strains of parvovirus have been identified from bat guano in the US states of Texas and California. Serum analysis of the straw-colored fruit bat andJamaican fruit batled to the identification of two new parvoviruses. Bat parvoviruses are in the subfamilyParvovirinae,closely resembling the generaProtoparvovirus,Erythrovirus,andBocaparvovirus.[18]
Double-stranded RNA viruses
[edit]Reoviruses
[edit]| Virus name | Year identified | Host | Location |
|---|---|---|---|
| Nelson Bay virus | 1968 | Bat | Australia |
| Pulau virus | 1999 | Bat | Malaysia |
| Melaka virus | 2006 | Human | Malaysia |
| Kampar virus | 2006 | Human | Malaysia |
| HK23629/07 | 2007 | Human | Hong Kong |
| Miyazaki-Bali/2007 | 2007 | Human | Indonesia/Japan |
| Sikamat virus | 2010 | Human | Malaysia |
| Xi River virus | 2010 | Bat | China |
| Indonesia/2010 | 2010 | Bat | Indonesia/Italy |
Zoonotic
[edit]Some disease-causing reovirus species are associated with bats. One such virus isMelaka virus,which was linked to illness in a Malaysian man and his two children in 2006.[25][26]The man said that a bat had been in his home a week before he became ill, and the virus was closely related to other reoviruses linked to bats.Kampar viruswas identified a few months later in another Malaysian man. Though he had no known contact with bats, Kampar virus is closely related to Melaka virus. Several other reovirus strains identified in ill humans are known as Miyazaki-Bali/2007,Sikamat virus,and SI-MRV01. No reoviruses linked to bats have caused death in humans.[25]
Other
[edit]Reoviruses include many viruses that do not cause disease in humans, including several found in bats. One reovirus species associated with bats isNelson Bay orthoreovirus,sometimes calledPteropine orthoreovirus(PRV), which is anorthoreovirus;several virus strains of it have been identified in bats. The type member ofNelson Bay orthoreovirusis Nelson Bay virus (NBV), which was first identified in 1970 from the blood of agray-headed flying foxinNew South Wales,Australia. NBV was the first reovirus to be isolated from a bat species. Another strain ofNelson Bay orthoreovirusassociated with bats isPulau virus,which was first identified from thesmall flying foxofTioman Islandin 2006. Other viruses includeBroome orthoreovirusfrom thelittle red flying foxofBroome,Western Australia;Xi River virusfromLeschenault's rousetteinGuangdong,China; andCangyuan virusalso from Leschenault's rousette.[25]Severalmammalian orthoreovirusesare associated with bats, including at least three from Germany and 19 from Italy. These were found inpipistrelles,thebrown long-eared bat,and thewhiskered bat.[25]
Orbiviruseshave been isolated from bats, includingIfe virusfrom the straw-colored fruit bat,Japanaut virusfrom thecommon blossom bat,andFomédé virusfromNycterisspecies.[25]
Positive-sense single-stranded RNA viruses
[edit]Astroviruses
[edit]Astroviruseshave been found in several genera of bat in theOld World,includingMiniopterus,Myotis,Hipposideros,Rhinolophus,Pipistrellus,Scotophilus,andTaphozous,[18]though none in Africa.[7]Bats have very high prevalence rates of astroviruses; studies in Hong Kong and mainland China found prevalence rates approaching 50% from anal swabs. No astroviruses identified in bats are associated with disease in humans.[18]
Caliciviruses
[edit]Batcaliciviruseswere first identified inHong Kongin thePomona roundleaf bat,[18]and were later identified fromtricolored batsin the US state of Maryland. Bat caliciviruses are similar to the generaSapovirusandValovirus,withnorovirusesalso detected from two microbat species in China.[27]
Coronaviruses
[edit]SARS-CoV, SARS-CoV-2, and MERS-CoV
[edit]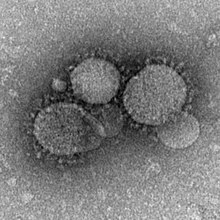
Several zoonotic coronaviruses are associated with bats, including severe acute respiratory syndrome coronavirus (SARS-CoV) andMiddle East respiratory syndrome-related coronavirus(MERS-CoV).[28]Severe acute respiratory syndrome coronavirus 2is another zoonotic coronavirus likely originating in bats.[29][30]SARS-CoV causes the diseasesevere acute respiratory syndrome(SARS) in humans. The first documented case of SARS was in November 2002 inFoshan,China.[28]It became anepidemic,affecting 28 countries around the world with 8,096 cases and 774 deaths.[28]The natural reservoir of SARS-CoV was identified as bats, with theChinese rufous horseshoe batconsidered a particularly strong candidate after a coronavirus was recovered from a colony that had 95% nucleotide sequence similarity to SARS-CoV.[28]There is uncertainty on whether or not animals likeHimalayan palm civetsandraccoon dogswere intermediate hosts that facilitated the spread of the virus from bats to humans, or if humans acquired the virus directly from bats.[28][31]
The first human case ofMiddle East respiratory syndrome(MERS) was in June 2012 inJeddah,Saudi Arabia.[28]As of November 2019, 2,494 cases of MERS have been reported in twenty-seven countries, resulting in 858 fatalities.[32]It is believed that MERS-CoV originated in bats, thoughcamelsare likely the intermediate host through which humans became infected. Human-to-human transmission is possible, though does not easily occur.[33]
TheCOVID-19 pandemicin humans started inWuhan,China in 2019.[34]Genetic analyses of SARS-COV-2 showed that it was highly similar to viruses found in horseshoe bats, with 96% similarity to a virus isolated from theintermediate horseshoe bat.Due to similarity with known bat coronaviruses, data "clearly indicates" that the natural reservoirs of SARS-COV-2 are bats. It is yet unclear how the virus was transmitted to humans, though an intermediate host may have been involved.[3]Phylogenetic reconstruction of SARS-CoV-2 suggests that the strain that caused a human pandemic diverged from the strain found in bats decades ago, likely between 1950 and 1980.[35]
Other
[edit]Bats harbor a great diversity ofcoronaviruses,with sampling by theEcoHealth Alliancein China alone identifying about 400 new strains of coronavirus.[36]A study of coronavirus diversity harbored by bats in eastern Thailand revealed forty-seven coronaviruses.[37]
Flaviviruses
[edit]
Mostflavivirusesare transmitted via arthropods, but bats may play a role in the ecology of some species. Several strains ofDengue virushave been found in bats in the Americas, andWest Nile virushas been identified in fruit bats in South India. Serological studies indicate thatWest Nile virusmay also be present in bats in North America and theYucatán Peninsula.Saint Louis encephalitis virushas been detected in bats in the US states of Texas and Ohio, as well as the Yucatán Peninsula.Japanese encephalitis virusor its associated antibodies have been found in several bat species throughout Asia. Other flaviviruses detected in bats includeSepik virus,Entebbe bat virus,Sokuluk virus,Yokose virus,Dakar bat virus,Bukalasa bat virus,Carey Island virus,Phnom Penh bat virus,Rio Bravo bat virus,Montana myotis leukoencephalitis virus,andTamana bat virus.[18]
Picornaviruses
[edit]Several genera ofpicornaviruseshave been found in bats, includingKobuvirus,Sapelovirus,Cardiovirus,andSenecavirus.[18]Picornaviruses have been identified from a diverse array of bat species around the world.[7]
Negative-sense single-stranded RNA viruses
[edit]Arenaviruses
[edit]Arenavirusesare mainly associated withrodents,though some can cause illness in humans. The first arenavirus identified in bats wasTacaribe mammarenavirus,which was isolated from Jamaican fruit bats and thegreat fruit-eating bat.Antibody response associated with Tacaribe virus has also been found in the common vampire bat, thelittle yellow-shouldered bat,andHeller's broad-nosed bat.It is unclear if bats are the natural reservoir of Tacaribe virus. There has been one known human infection by Tacaribe virus, though it was accidentally acquired in a laboratory setting.[18]
Hantaviruses
[edit]Hantaviruses,familyHantaviridae,naturally occur in vertebrates. All bat-associated hantaviruses are in the subfamilyMammantavirinae.Of the four genera within the subfamily,LoanvirusandMobatvirusare the genera that have been documented in various bats. Almost all bat hantaviruses have been identified from microbats.[38]Mouyassue virushas been identified from thebanana pipistrelleinIvory Coastand theCape serotinein Ethiopia;[38]Magboi virusfrom thehairy slit-faced batinSierra Leone;Xuan Son virusfrom the Pomona roundleaf bat in Vietnam;Huangpi virusfrom theJapanese house batin China;Longquan loanvirusfrom several horseshoe bats in China;[18]Makokou virusfromNoack's roundleaf batin Gabon;Đakrông virusfromStoliczka's trident batin Vietnam;[38]Brno loanvirusfrom thecommon noctulein the Czech Republic;[38]andLaibin mobatvirusfrom theblack-bearded tomb batin China.[39]As of 2019, onlyQuezon mobatvirushas been identified from a megabat, as it was identified from aGeoffroy's rousettein the Philippines.[38]Bat hantaviruses are not associated with illness in humans.[18][38]
Filoviruses
[edit]MarburgvirusandEbolavirus
[edit]
Filoviridaeis a family of virus containing two genera associated with bats:MarburgvirusandEbolavirus,which contain the species that causeMarburg virus diseaseandEbola virus disease,respectively. Though relatively few disease outbreaks are caused by filoviruses, they are of high concern due to their extremevirulence,or capacity to cause harm to their hosts. Filovirus outbreaks typically have high mortality rates in humans. Though the first filovirus was identified in 1967, it took more than twenty years to identify any natural reservoirs.[40]
Ebola virus disease is a relatively rare but life-threatening illness in humans, with an average mortality rate of 50% (though individual outbreaks may be as high as 90% mortality). The first outbreaks were in 1976 inSouth SudanandDemocratic Republic of the Congo.[41]The natural reservoirs of ebolaviruses are unknown.[42][43][44]However, some evidence indicates that megabats may be natural reservoirs.[40][41]Several megabat species have testedseropositiveforantibodiesagainst ebolaviruses, including thehammer-headed bat,Franquet's epauletted fruit bat,andlittle collared fruit bat.[40]Among others, it has been posited that theWestern African Ebola virus epidemicbegan with a spillover event from anAngolan free-tailed batto a human.[45]Other possible reservoirs include non-humanprimates,[42]rodents, shrews, carnivores, and ungulates.[46]Definitively stating that fruit bats are natural reservoirs is problematic; as of 2017, researchers have been largely unable to isolate ebolaviruses or their viral RNA sequences from fruit bats. Additionally, bats typically have low level of ebolavirus-associated antibodies, and seropositivity in bats is not strongly correlated to human outbreaks.[44]
Marburg virus disease (MVD) was first identified in 1967 during simultaneous outbreaks inMarburgandFrankfurtin Germany, andBelgrade, Serbia.MVD is highly virulent, with an average human mortality rate of 50%, but as high as 88% for individual outbreaks.[47]MVD is caused byMarburg virusand the closely relatedRavn virus,which was formerly considered synonymous with Marburg virus.[48]Marburg virus was first detected in theEgyptian fruit batin 2007,[40]which is now recognized as the natural reservoir of the virus.[47]Marburg virus has been detected in Egyptian fruit bats in Gabon, Democratic Republic of the Congo, Kenya, and Uganda.[40]Spillover from Egyptian fruit bats occurs when humans spend prolonged time in mines or caves inhabited by the bats,[47]though the exact mechanism of transmission is unclear.[40]Human-to-human transmission occurs through direct contact with infected bodily fluids, including blood or semen, or indirectly through contact with bedding or clothing exposed to these fluids.[47]
Other
[edit]Lloviu virus,a kind of filovirus in the genusCuevavirus,has been identified from thecommon bent-wing batin Spain.[40]Another filovirus,Bombali ebolavirus,has been isolated fromfree-tailed bats,including thelittle free-tailed batand theAngolan free-tailed bat.[49]Neither Lloviu virus norBombali ebolavirusis associated with illness in humans.[50][49]Genomic RNA associated withMengla dianlovirus,though not the virus itself, has been identified fromRousettusbats in China.[49]
Rhabdoviruses
[edit]Rabies-causing viruses
[edit]
Lyssaviruses(from the genusLyssavirusin the familyRhabdoviridae) include therabies virus,Australian bat lyssavirus,and other related viruses, many of which are also harbored by bats. Unlike most other viruses in the familyRhabdoviridae,which are transmitted by arthropods, lyssaviruses are transmitted by mammals, most frequently through biting. All mammals are susceptible to lyssaviruses, though bats and carnivores are the most common natural reservoirs. The vast majority of human rabies cases are a result of the rabies virus, with only twelve other human cases attributed to other lyssaviruses as of 2015.[51]These rarer lyssaviruses associated with bats includeDuvenhage lyssavirus(three human cases as of 2015);European bat 1 lyssavirus(one human case as of 2015);European bat 2 lyssavirus(two human cases as of 2015); andIrkut lyssavirus(one human case as of 2015). Microbats are suspected as the reservoirs of these four uncommon lyssaviruses.[51][52]
After transmission has occurred, the average human is asymptomatic for two months, though the incubation period can be as short as a week or as long as several years.[51] Italian scientistAntonio Cariniwas the first to hypothesize that rabies virus could be transmitted by bats, which he did in 1911. This same conclusion was reached byHélder Queirozin 1934 andJoseph Lennox Pawanin 1936.Vampire batswere the first to be documented with rabies; in 1953, an insectivorous bat in Florida was discovered with rabies, making it the first documented occurrence in an insectivorous species outside the vampire bats' ranges.[53]Bats have an overall low prevalence of rabies virus, with a majority of surveys of apparently healthy individuals showing rabies incidence of 0.0–0.5%.[51]Sick bats are more likely to be submitted for rabies testing than apparently healthy bats, known as sampling bias,[54]with most studies reporting rabies incidence of 5–20% in sick or dead bats.[51]Rabies virus exposure can be fatal in bats, though it is likely that the majority of individuals do not develop the disease after exposure.[51]In non-bat mammals, exposure to the rabies virus almost always leads to death.[52]

Globally, dogs are by far the most common source of human rabies deaths.[55]Bats are the most common source of rabies in humans in North and South America, Western Europe, and Australia.[56]Manyfeeding guildsof bats may transmit rabies to humans, including insectivorous, frugivorous, nectarivorous, omnivorous, sanguivorous, and carnivorous species.[56]The common vampire bat is a source of human rabies in Central and South America, though the frequency at which humans are bitten is poorly understood.[57]Between 1993 and 2002, the majority of human rabies cases associated with bats in the Americas were the result of non-vampire bats.[52]In North America, about half of human rabies instances arecryptic,meaning that the patient has no known bite history.[51]While it has been speculated that rabies virus could be transmitted through aerosols, studies of the rabies virus have concluded that this is only feasible in limited conditions. These conditions include a very large colony of bats in a hot and humid cave with poor ventilation. While two human deaths in 1956 and 1959 had been tentatively attributed to aerosolization of the rabies virus after entering a cave with bats, "investigations of the 2 reported human cases revealed that both infections could be explained by means other than aerosol transmission".[58]It is instead generally thought that most instances of cryptic rabies are the result of an unknown bat bite.[51]Bites from a bat can be so small that they are not visible without magnification equipment, for example. Outside of bites, rabies virus exposure can also occur if infected fluids come in contact with amucous membraneor a break in the skin.[58]
Other
[edit]Many bat lyssaviruses are not associated with infection in humans. These includeLagos bat lyssavirus,Shimoni bat lyssavirus,Khujand lyssavirus,Aravan lyssavirus,Bokeloh bat lyssavirus,West Caucasian bat lyssavirus,andLleida bat lyssavirus.[52][51]Lagos bat lyssavirus,also known as Lagos bat virus (LBV), has been isolated from a megabat in sub-Saharan Africa.[51]This lyssavirus has four distinct lineages, all of which are found in the straw-colored fruit bat.[59]
Rhabdoviruses from other genera have been identified in bats. This includes several from the genusLedantevirus:Kern Canyon virus,which was found in theYuma myotisin California (US);Kolente virusfrom theJones's roundleaf batin Guinea;[60]Mount Elgon bat virusfrom theeloquent horseshoe batin Kenya;Oita virusfrom thelittle Japanese horseshoe bat;andFikirini virusfrom thestriped leaf-nosed batin Kenya.[61]
Orthomyxoviruses
[edit]
Orthomyxovirusesincludeinfluenzaviruses. While birds are the primary reservoir for the genusAlphainfluenzavirus,a few bat species in Central and South America have also tested positive for the viruses. These species include the little yellow-shouldered bat and theflat-faced fruit-eating bat.Bat populations tested in Guatemala and Peru had high seropositivity rates, which suggests that influenza A infections are common among bats in the New World.[18]
Paramyxoviruses
[edit]Hendra, Nipah, and Menangle viruses
[edit]
Paramyxoviridaeis a family that includes several zoonotic viruses naturally found in bats. Two are in the genusHenipavirus—Hendra virusandNipah virus.Hendra virus was first identified in 1994 inHendra,Australia. Four different species offlying foxhave tested positive for Hendra virus: the gray-headed flying fox, little red flying fox,spectacled flying fox,andblack flying fox.[62]Horses are the intermediate host between flying foxes and humans. Between 1994 and 2014, there were fifty-five outbreaks of Hendra virus in Australia, resulting in the death or euthanization of eighty-eight horses. Seven humans are known to have been infected by Hendra virus, with four fatalities.[16]Six of the seven infected humans were directly exposed to the blood or other fluids of sick or dead horses (three were veterinarians), while the seventh case was a veterinary nurse who had recently irrigated the nasal cavity of a horse not yet exhibiting symptoms. It is unclear how horses become infected with Hendra virus, though it is believed to occur following direct exposure to flying fox fluids. There is also evidence of horse-to-horse transmission. In late 2012, avaccinewas released to prevent infection in horses.[62]Vaccine uptake has been low, with an estimated 11–17% of Australian horses vaccinated by 2017.[63]
The first human outbreak of Nipah virus was in 1998 in Malaysia.[16]It was determined that flying foxes were also the reservoir of the virus, with domestic pigs as the intermediate host between bats and humans. Outbreaks have also occurred in Bangladesh, India, Singapore, and the Philippines. In Bangladesh, the primary mode of transmission of Nipah virus to humans is through the consumption ofdate palm sap.Pots set out to collect the sap are contaminated with flying fox urine and guano, and the bats also lick the sap streams flowing into the pots. It has been speculated that the virus may also be transmitted to humans by eating fruit partially consumed by flying foxes, or by coming into contact with their urine, though no definitive evidence supports this.[64]
An additional zoonotic paramyxovirus that bats harbor isMenangle virus,which was first identified at a hog farm inNew South Wales,Australia. Flying foxes were once again identified as the natural reservoirs of the virus, with the black, spectacled, and gray-headedseropositivefor the virus. Two employees of the hog farm became sick with flu-like illnesses, later shown to be a result of the virus.[16]Sosuga pararubulavirusis known to have infected one person—an American wildlife biologist who had conducted bat and rodent research in Uganda.[16]TheEgyptian fruit batlater tested positive for the virus, indicating that it is potentially a natural reservoir.[65]
Other
[edit]Bats host several paramyxoviruses that are not known to affect humans. Bats are the reservoir ofCedar virus,a paramyxovirus first discovered in flying foxesSouth East Queensland.[16]The zoonotic potential of Cedar virus is unknown.[66]In Brazil in 1979,Mapuera orthorubulaviruswas isolated from the saliva of the little yellow-shouldered bat. Mapuera virus has never been associated with disease in other animals or humans, but experimental exposure of mice to the virus resulted in fatality.[16]Tioman pararubulavirushas been isolated from the urine of the small flying fox, which causes fever in some domestic pigs after exposure, but no other symptoms.Tukoko virushas been detected from Leschenault's rousette in China.[16]Bats have been suggested as the host ofPorcine orthorubulavirus,though definitive evidence has not been collected.[16]
Togaviruses
[edit]Togavirusesincludealphaviruses,which have been detected in bats. Alphaviruses causeencephalitisin humans. Alphaviruses that have been detected in bats includeVenezuelan equine encephalitis virus,Eastern equine encephalitis virus,andWestern equine encephalitis virus.Sindbis virushas been detected from horseshoe bats androundleaf bats.Chikungunya virushas been isolated from Leschenault's rousette, the Egyptian fruit bat,Sundevall's roundleaf bat,the little free-tailed bat, andScotophilusspecies.[18]
Positive-sense single-stranded RNA viruses that replicate through a DNA intermediate
[edit]Retroviruses
[edit]Bats can be infected withretroviruses,including thegammaretrovirusfound in horseshoe bats, Leschenault's rousette, and thegreater false vampire bat.Several bat retroviruses have been identified that are similar to theReticuloendotheliosis virusfound in birds. These retroviruses were found inmouse-eared bats,horseshoe bats, and flying foxes. The discovery of varied and distinct gammaretroviruses in bat genomes indicates that bats likely played important roles in their diversification. Bats also host an extensive number ofbetaretroviruses,including within mouse-eared bats, horseshoe bats, and flying foxes. Bat betaretroviruses span the entire breadth of betaretrovirus diversity, similar to those of rodents, which may indicate that bats and rodents are primary reservoirs of the viruses. Betaretroviruses have infected bats for a majority of bat evolutionary history, since at least 36 million years ago.[67]
Double-stranded DNA viruses that replicate through a single-stranded RNA intermediate
[edit]
Hepadnaviruses
[edit]Hepadnavirusesare also known to affect bats, with thetent-making bat,Noack's roundleaf bat, and thehalcyon horseshoe batknown to harbor several. The hepadnavirus found in the tent-making bat, which is a New World species, was the closest relative of human hepadnaviruses.[67]Though relatively few hepadnaviruses have been identified in bats, it is highly likely that additional strains will be discovered through further research. As of 2016, they had been found in four bat families:HipposideridaeandRhinolophidaefrom the suborderYinpterochiropteraandMolossidaeandVespertilionidaefromYangochiroptera.The high diversity of bat hosts suggests that bats share a long evolutionary history with hepadnaviruses, indicating bats may have had an important role in hepadnavirus evolution.[68]
See also
[edit]References
[edit]- ^abCalisher, C. H.;Childs, J. E.; Field, H. E.; Holmes, K. V.; Schountz, T. (2006)."Bats: Important Reservoir Hosts of Emerging Viruses".Clinical Microbiology Reviews.19(3): 531–545.doi:10.1128/CMR.00017-06.PMC1539106.PMID16847084.
- ^Moratelli, Ricardo; Calisher, Charles H. (2015)."Bats and zoonotic viruses: Can we confidently link bats with emerging deadly viruses?".Memórias do Instituto Oswaldo Cruz.110(1): 1–22.doi:10.1590/0074-02760150048.PMC4371215.PMID25742261.
An increasingly asked question is 'can we confidently link bats with emerging viruses?'. No, or not yet, is the qualified answer based on the evidence available.
- ^abMacKenzie, John S.; Smith, David W. (2020)."COVID-19: A novel zoonotic disease caused by a coronavirus from China: What we know and what we don't".Microbiology Australia.41:45.doi:10.1071/MA20013.PMC7086482.PMID32226946.
Evidence from the sequence analyses clearly indicates that the reservoir host of the virus was a bat, probably a Chinese or Intermediate horseshoe bat, and it is probable that, like SARS-CoV, an intermediate host was the source of the outbreak.
- ^abOlival, Kevin J.; Weekley, Cristin C.; Daszak, Peter (2015). "Are Bats Really 'Special' as Viral Reservoirs? What We Know and Need to Know".Bats and Viruses.pp. 281–294.doi:10.1002/9781118818824.ch11.ISBN978-1118818824.
- ^abMollentze, Nardus; Streicker, Daniel G. (2020)."Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts".Proceedings of the National Academy of Sciences.117(17): 9423–9430.Bibcode:2020PNAS..117.9423M.doi:10.1073/pnas.1919176117.PMC7196766.PMID32284401.
- ^abcLetko, Michael; Seifert, Stephanie N.; Olival, Kevin J.; Plowright, Raina K.; Munster, Vincent J. (2020)."Bat-borne virus diversity, spillover and emergence".Nature Reviews Microbiology.18(8): 461–471.doi:10.1038/s41579-020-0394-z.PMC7289071.PMID32528128.
- ^abcdefghiHayman, David T.S. (2016)."Bats as Viral Reservoirs".Annual Review of Virology.3(1): 77–99.doi:10.1146/annurev-virology-110615-042203.PMID27578437.
- ^abcdeXie, Jiazheng; Li, Yang; Shen, Xurui; Goh, Geraldine; Zhu, Yan; Cui, Jie; Wang, Lin-Fa; Shi, Zheng-Li; Zhou, Peng (2018)."Dampened STING-Dependent Interferon Activation in Bats".Cell Host & Microbe.23(3): 297–301.e4.doi:10.1016/j.chom.2018.01.006.PMC7104992.PMID29478775.
- ^Gorman, James (28 January 2020)."How Do Bats Live With So Many Viruses?".The New York Times.Retrieved17 March2020.
- ^Kuno, Goro (2001). "Persistence of arboviruses and antiviral antibodies in vertebrate hosts: its occurrence and impacts".Reviews in Medical Virology.11(3): 165–190.doi:10.1002/rmv.314.PMID11376480.S2CID22591717.
- ^Sarkar, Saurav K.; Chakravarty, Ashim K. (1991). "Analysis of immunocompetent cells in the bat, Pteropus giganteus: Isolation and scanning electron microscopic characterization".Developmental & Comparative Immunology.15(4): 423–430.doi:10.1016/0145-305x(91)90034-v.PMID1773865.
- ^Barber, Glen N. (2015)."STING: Infection, inflammation and cancer".Nature Reviews Immunology.15(12): 760–770.doi:10.1038/nri3921.PMC5004891.PMID26603901.
- ^Ahn, Matae; Anderson, Danielle E.; Zhang, Qian; Tan, Chee Wah; Lim, Beng Lee; Luko, Katarina; Wen, Ming; Chia, Wan Ni; Mani, Shailendra; Wang, Loo Chien; et al. (2019)."Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host".Nature Microbiology.4(5): 789–799.doi:10.1038/s41564-019-0371-3.PMC7096966.PMID30804542.
- ^Yong, Kylie Su Mei; Ng, Justin Han Jia; Her, Zhisheng; Hey, Ying Ying; Tan, Sue Yee; Tan, Wilson Wei Sheng; Irac, Sergio Erdal; Liu, Min; Chan, Xue Ying; Gunawan, Merry; et al. (2018)."Bat-mouse bone marrow chimera: A novel animal model for dissecting the uniqueness of the bat immune system".Scientific Reports.8(1): 4726.Bibcode:2018NatSR...8.4726Y.doi:10.1038/s41598-018-22899-1.PMC5856848.PMID29549333.
- ^abcJoffrin, Léa; Dietrich, Muriel; Mavingui, Patrick; Lebarbenchon, Camille (2018)."Bat pathogens hit the road: But which one?".PLOS Pathogens.14(8): e1007134.doi:10.1371/journal.ppat.1007134.PMC6085074.PMID30092093.
- ^abcdefghiAnderson, Danielle E.; Marsh, Glenn A. (2015). "Bat Paramyxoviruses".Bats and Viruses.pp. 99–126.doi:10.1002/9781118818824.ch4.ISBN978-1118818824.
- ^Young, Cristin C. W.; Olival, Kevin J. (2016)."Optimizing Viral Discovery in Bats".PLOS ONE.11(2): e0149237.Bibcode:2016PLoSO..1149237Y.doi:10.1371/journal.pone.0149237.PMC4750870.PMID26867024.
- ^abcdefghijklmnQueen, Krista; Shi, Mang; Anderson, Larry J.; Tong, Suxiang (2015). "Other Bat-Borne Viruses".Bats and Viruses.pp. 217–247.doi:10.1002/9781118818824.ch9.ISBN9781118818824.
- ^"ICTV Master Species List 2018b.v2".International Committee on Taxonomy of Viruses (ICTV).Archived fromthe originalon 30 March 2019.Retrieved19 June2019.
- ^Cibulski, S. P.; Teixeira, T. F.; De Sales Lima, F. E.; Do Santos, H. F.; Franco, A. C.; Roehe, P. M. (2014)."A Novel Anelloviridae Species Detected in Tadarida brasiliensis Bats: First Sequence of a Chiropteran Anellovirus".Genome Announcements.2(5).doi:10.1128/genomeA.01028-14.PMC4214982.PMID25359906.
- ^De Souza, William Marciel; Fumagalli, Marcílio Jorge; De Araujo, Jansen; Sabino-Santos, Gilberto; Maia, Felipe Gonçalves Motta; Romeiro, Marilia Farignoli; Modha, Sejal; Nardi, Marcello Schiavo; Queiroz, Luzia Helena; Durigon, Edison Luiz; et al. (2018)."Discovery of novel anelloviruses in small mammals expands the host range and diversity of the Anelloviridae".Virology.514:9–17.doi:10.1016/j.virol.2017.11.001.hdl:11449/165970.PMID29128758.
- ^abLecis, Roberta; Mucedda, Mauro; Pidinchedda, Ermanno; Zobba, Rosanna; Pittau, Marco; Alberti, Alberto (2020)."Genomic characterization of a novel bat-associated Circovirus detected in European Miniopterus schreibersii bats".Virus Genes.56(3): 325–328.doi:10.1007/s11262-020-01747-3.PMC7088871.PMID32088806.
- ^Han, H.-J.; Wen, H.-L.; Zhao, L.; Liu, J.-W.; Luo, L.-M.; Zhou, C.-M.; Qin, X.-R.; Zhu, Y.-L.; Liu, M.-M.; Qi, R.; et al. (2017)."Novel coronaviruses, astroviruses, adenoviruses and circoviruses in insectivorous bats from northern China".Zoonoses and Public Health.64(8): 636–646.doi:10.1111/zph.12358.PMC7165899.PMID28371451.
- ^Lorusso, Alessio; Teodori, Liana; Leone, Alessandra; Marcacci, Maurilia; Mangone, Iolanda; Orsini, Massimiliano; Capobianco-Dondona, Andrea; Camma’, Cesare; Monaco, Federica; Savini, Giovanni (2015)."A new member of the Pteropine Orthoreovirus species isolated from fruit bats imported to Italy".Infection, Genetics and Evolution.30:55–58.doi:10.1016/j.meegid.2014.12.006.PMID25497353.
- ^abcdeKohl, Claudia; Kurth, Andreas (2015). "Bat Reoviruses".Bats and Viruses.pp. 203–215.doi:10.1002/9781118818824.ch8.ISBN9781118818824.
- ^Tan, Yeh Fong; Teng, Cheong Lieng; Chua, Kaw Bing; Voon, Kenny (2017)."Pteropine orthoreovirus: An important emerging virus causing infectious disease in the tropics?".The Journal of Infection in Developing Countries.11(3): 215–219.doi:10.3855/jidc.9112.PMID28368854.
- ^Kocher, Jacob F.; Lindesmith, Lisa C.; Debbink, Kari; Beall, Anne; Mallory, Michael L.; Yount, Boyd L.; Graham, Rachel L.; Huynh, Jeremy; Gates, J. Edward; Donaldson, Eric F.; et al. (2018)."Bat Caliciviruses and Human Noroviruses Are Antigenically Similar and Have Overlapping Histo-Blood Group Antigen Binding Profiles".mBio.9(3).doi:10.1128/mBio.00869-18.PMC5964351.PMID29789360.
- ^abcdefGe, Xing-Yi; Hu, Ben; Shi, Zheng-Li (2015). "Bat Coronaviruses".Bats and Viruses.pp. 127–155.doi:10.1002/9781118818824.ch5.ISBN978-1118818824.
- ^Zhou, Peng; Yang, Xing-Lou; Wang, Xian-Guang; Hu, Ben; Zhang, Lei; Zhang, Wei; Si, Hao-Rui; Zhu, Yan; Li, Bei; Huang, Chao-Lin; et al. (2020)."A pneumonia outbreak associated with a new coronavirus of probable bat origin".Nature.579(7798): 270–273.Bibcode:2020Natur.579..270Z.doi:10.1038/s41586-020-2012-7.PMC7095418.PMID32015507.
- ^"Novel Coronavirus (2019-nCoV) Situation Report – 22"(PDF).World Health Organization.11 February 2020.Retrieved15 February2020.
- ^Lu, Guangwen; Wang, Qihui; Gao, George F. (2015)."Bat-to-human: Spike features determining 'host jump' of coronaviruses SARS-CoV, MERS-CoV, and beyond".Trends in Microbiology.23(8): 468–478.doi:10.1016/j.tim.2015.06.003.PMC7125587.PMID26206723.
- ^"Middle East respiratory syndrome coronavirus (MERS-CoV)".World Health Organization.November 2019.Retrieved5 April2020.
- ^"Middle East respiratory syndrome coronavirus (MERS-CoV)".World Health Organization.11 March 2019.Retrieved5 April2020.
- ^Nsikan, Akpan (21 January 2020)."New coronavirus can spread between humans – but it started in a wildlife market".National Geographic.Archived fromthe originalon 22 January 2020.Retrieved23 January2020.
- ^Fenton, M. Brock; Mubareka, Samira; Tsang, Susan M.; Simmons, Nancy B.; Becker, Daniel J. (2020)."COVID-19 and threats to bats".Facets.5:349–352.doi:10.1139/facets-2020-0028.
- ^Aizenman, Nurith (20 February 2020)."New Research: Bats Harbor Hundreds Of Coronaviruses, And Spillovers Aren't Rare".NPR.Retrieved5 April2020.
- ^Wacharapluesadee, Supaporn; Duengkae, Prateep; Rodpan, Apaporn; Kaewpom, Thongchai; Maneeorn, Patarapol; Kanchanasaka, Budsabong; Yingsakmongkon, Sangchai; Sittidetboripat, Nuntaporn; Chareesaen, Chaiyaporn; Khlangsap, Nathawat; et al. (2015)."Diversity of coronavirus in bats from Eastern Thailand".Virology Journal.12:57.doi:10.1186/s12985-015-0289-1.PMC4416284.PMID25884446.
- ^abcdefArai, Satoru; Aoki, Keita; Sơn, Nguyễn Trường; Tú, Vương Tân; Kikuchi, Fuka; Kinoshita, Gohta; Fukui, Dai; Thành, Hoàng Trung; Gu, Se Hun; Yoshikawa, Yasuhiro; et al. (2019)."Đakrông virus, a novel mobatvirus (Hantaviridae) harbored by the Stoliczka's Asian trident bat (Aselliscus stoliczkanus) in Vietnam".Scientific Reports.9(1): 10239.Bibcode:2019NatSR...910239A.doi:10.1038/s41598-019-46697-5.PMC6629698.PMID31308502.
- ^Xu, Lin; Wu, Jianmin; He, Biao; Qin, Shaomin; Xia, Lele; Qin, Minchao; Li, Nan; Tu, Changchun (2015)."Novel hantavirus identified in black-bearded tomb bats, China".Infection, Genetics and Evolution.31:158–160.doi:10.1016/j.meegid.2015.01.018.PMC7172206.PMID25643870.
- ^abcdefgMaganga, Gael Darren; Rougeron, Virginie; Leroy, Eric Maurice (2015). "Bat Filoviruses".Bats and Viruses.pp. 157–175.doi:10.1002/9781118818824.ch6.ISBN9781118818824.
- ^ab"Ebola virus disease".World Health Organization.10 February 2020.Retrieved13 April2020.
- ^ab"What is Ebola Virus Disease?".Centers for Disease Control and Prevention.5 November 2019.Retrieved13 April2020.
Scientists do not know where Ebola virus comes from.
- ^Rewar, Suresh; Mirdha, Dashrath (2015)."Transmission of Ebola Virus Disease: An Overview".Annals of Global Health.80(6): 444–451.doi:10.1016/j.aogh.2015.02.005.PMID25960093.
Despite concerted investigative efforts, the natural reservoir of the virus is unknown.
- ^abBaseler, Laura; Chertow, Daniel S.; Johnson, Karl M.; Feldmann, Heinz; Morens, David M. (2017)."The Pathogenesis of Ebola Virus Disease".Annual Review of Pathology: Mechanisms of Disease.12:387–418.doi:10.1146/annurev-pathol-052016-100506.PMID27959626.
The geographic ranges of many animal species, including bats, squirrels, mice and rats, dormice, and shrews, match or overlap with known outbreak sites of African filoviruses, but none of these mammals has yet been universally accepted as an EBOV reservoir.
- ^von Csefalvay, Chris (2023),"Host-vector and multihost systems",Computational Modeling of Infectious Disease,Elsevier, pp. 121–149,doi:10.1016/b978-0-32-395389-4.00013-x,ISBN978-0-323-95389-4,retrieved2 March2023
- ^Olivero, Jesús; Fa, John E.; Real, Raimundo; Farfán, Miguel Ángel; Márquez, Ana Luz; Vargas, J. Mario; Gonzalez, J. Paul; Cunningham, Andrew A.; Nasi, Robert (2017)."Mammalian biogeography and the Ebola virus in Africa"(PDF).Mammal Review.47:24–37.doi:10.1111/mam.12074.
We found published evidence from cases of serological and/or polymerase chain reaction (PCR) positivity of EVD in non- human mammal, or of EVD-linked mortality, in 28 mammal species: 10 primates, three rodents, one shrew, eight bats, one carnivore, and five ungulates
- ^abcd"Marburg virus disease".World Health Organization.15 February 2018.Retrieved14 April2020.
- ^Yang, Xing-Lou; Tan, Chee Wah; Anderson, Danielle E.; Jiang, Ren-Di; Li, Bei; Zhang, Wei; Zhu, Yan; Lim, Xiao Fang; Zhou, Peng; Liu, Xiang-Ling; et al. (2019). "Characterization of a filovirus (Měnglà virus) from Rousettus bats in China".Nature Microbiology.4(3): 390–395.doi:10.1038/s41564-018-0328-y.PMID30617348.S2CID57574565.
- ^abc"Filoviruses – Ebola and Marburg Viruses".BU Research Support.12 June 2019.Retrieved14 April2020.
- ^Edwards, Megan R.; Basler, Christopher F. (2019)."Current status of small molecule drug development for Ebola virus and other filoviruses".Current Opinion in Virology.35:42–56.doi:10.1016/j.coviro.2019.03.001.PMC6556423.PMID31003196.
- ^abcdefghijKuzmin, Ivan V.; Rupprecht, Charles E. (2015). "Bat Lyssaviruses".Bats and Viruses.pp. 47–97.doi:10.1002/9781118818824.ch3.ISBN978-1118818824.
- ^abcdBanyard, Ashley C.; Hayman, David; Johnson, Nicholas; McElhinney, Lorraine; Fooks, Anthony R. (2011). "Bats and Lyssaviruses".Research Advances in Rabies.Advances in Virus Research. Vol. 79. pp. 239–289.doi:10.1016/B978-0-12-387040-7.00012-3.ISBN978-0123870407.PMID21601050.
- ^Calisher, Charles H. (2015). "Viruses in Bats".Bats and Viruses.pp. 23–45.doi:10.1002/9781118818824.ch2.ISBN978-1118818824.
- ^Klug, BJ; Turmelle, AS; Ellison, JA; Baerwald, EF; Barclay, RM (2010)."Rabies prevalence in migratory tree-bats in Alberta and the influence of roosting ecology and sampling method on reported prevalence of rabies in bats".Journal of Wildlife Diseases.47(1): 64–77.doi:10.7589/0090-3558-47.1.64.PMID21269998.
- ^"Rabies".www.who.int.Retrieved8 July2020.
- ^abCalderón, Alfonso; Guzmán, Camilo; Mattar, Salim; Rodríguez, Virginia; Acosta, Arles; Martínez, Caty (2019)."Frugivorous bats in the Colombian Caribbean region are reservoirs of the rabies virus".Annals of Clinical Microbiology and Antimicrobials.18(1): 11.doi:10.1186/s12941-019-0308-y.PMC6423830.PMID30890183.
- ^Brock Fenton, M.; Streicker, Daniel G.; Racey, Paul A.; Tuttle, Merlin D.; Medellin, Rodrigo A.; Daley, Mark J.; Recuenco, Sergio; Bakker, Kevin M. (2020)."Knowledge gaps about rabies transmission from vampire bats to humans".Nature Ecology & Evolution.4(4): 517–518.doi:10.1038/s41559-020-1144-3.PMC7896415.PMID32203471.S2CID212732288.
- ^abMessenger, Sharon L.; Smith, Jean S.; Rupprecht, Charles E. (2002)."Emerging Epidemiology of Bat-Associated Cryptic Cases of Rabies in Humans in the United States".Clinical Infectious Diseases.35(6): 738–747.doi:10.1086/342387.PMID12203172.
- ^Suu-Ire, Richard; Begeman, Lineke; Banyard, Ashley C.; Breed, Andrew C.; Drosten, Christian; Eggerbauer, Elisa; Freuling, Conrad M.; Gibson, Louise; Goharriz, Hooman; Horton, Daniel L.; et al. (2018)."Pathogenesis of bat rabies in a natural reservoir: Comparative susceptibility of the straw-colored fruit bat (Eidolon helvum) to three strains of Lagos bat virus".PLOS Neglected Tropical Diseases.12(3): e0006311.doi:10.1371/journal.pntd.0006311.PMC5854431.PMID29505617.
- ^Blasdell, Kim R.; Widen, Steven G.; Wood, Thomas G.; Holmes, Edward C.; Vasilakis, Nikos; Tesh, Robert B.; Walker, Peter J.; Guzman, Hilda; Firth, Cadhla (2015)."Ledantevirus: A Proposed New Genus in the Rhabdoviridae has a Strong Ecological Association with Bats".The American Journal of Tropical Medicine and Hygiene.92(2): 405–410.doi:10.4269/ajtmh.14-0606.PMC4347348.PMID25487727.
- ^Walker, Peter J.; Firth, Cadhla; Widen, Steven G.; Blasdell, Kim R.; Guzman, Hilda; Wood, Thomas G.; Paradkar, Prasad N.; Holmes, Edward C.; Tesh, Robert B.; Vasilakis, Nikos (2015)."Evolution of Genome Size and Complexity in the Rhabdoviridae".PLOS Pathogens.11(2): e1004664.doi:10.1371/journal.ppat.1004664.PMC4334499.PMID25679389.
- ^abMiddleton, Deborah (2014)."Hendra Virus".Veterinary Clinics of North America: Equine Practice.30(3): 579–589.doi:10.1016/j.cveq.2014.08.004.PMC4252762.PMID25281398.
- ^Manyweathers, J.; Field, H.; Longnecker, N.; Agho, K.; Smith, C.; Taylor, M. (2017).""Why won't they just vaccinate?" Horse owner risk perception and uptake of the Hendra virus vaccine ".BMC Veterinary Research.13(1): 103.doi:10.1186/s12917-017-1006-7.PMC5390447.PMID28407738.
- ^Aditi; Shariff, M. (2019)."Nipah virus infection: A review".Epidemiology and Infection.147:e95.doi:10.1017/S0950268819000086.PMC6518547.PMID30869046.
- ^Amman, Brian R.; Albariño, Cesar G.; Bird, Brian H.; Nyakarahuka, Luke; Sealy, Tara K.; Balinandi, Stephen; Schuh, Amy J.; Campbell, Shelly M.; Ströher, Ute; Jones, Megan E. B.; et al. (2015)."A Recently Discovered Pathogenic Paramyxovirus, Sosuga Virus, is Present in Rousettus aegyptiacus Fruit Bats at Multiple Locations in Uganda".Journal of Wildlife Diseases.51(3): 774–779.doi:10.7589/2015-02-044.PMC5022529.PMID25919464.
- ^Laing, Eric D.; Navaratnarajah, Chanakha K.; Cheliout Da Silva, Sofia; Petzing, Stephanie R.; Xu, Yan; Sterling, Spencer L.; Marsh, Glenn A.; Wang, Lin-Fa; Amaya, Moushimi; Nikolov, Dimitar B.; et al. (2019)."Structural and functional analyses reveal promiscuous and species specific use of ephrin receptors by Cedar virus".Proceedings of the National Academy of Sciences.116(41): 20707–20715.Bibcode:2019PNAS..11620707L.doi:10.1073/pnas.1911773116.PMC6789926.PMID31548390.
- ^abTachedjian, Gilda; Hayward, Joshua A.; Cui, Jie (2015). "Bats and Reverse Transcribing RNA and DNA Viruses".Bats and Viruses.pp. 177–201.doi:10.1002/9781118818824.ch7.ISBN9781118818824.
- ^Rasche, Andrea; Souza, Breno Frederico de Carvalho Dominguez; Drexler, Jan Felix (2016). "Bat hepadnaviruses and the origins of primate hepatitis B viruses".Current Opinion in Virology.16:86–94.doi:10.1016/j.coviro.2016.01.015.PMID26897577.
