Clomacran
Appearance
 | |
| Clinical data | |
|---|---|
| Trade names | Devryl, Olaxin,[1]Develar[2][3] |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
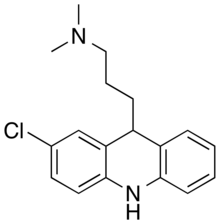
| Formula | C18H21ClN2 |
| Molar mass | 300.83g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.120 g/cm3g/cm3[1] |
| |
| |
Clomacranis anantipsychotic drugof the dihydroacridineclass, developed in the 1970s[2]by the pharmaceutical companySmith, Kline & French(nowGlaxoSmithKline) under the brand names Devryl and Olaxin.[1]
It was used to treat schizophrenia in the 1970s.[6]It was withdrawn from the market in the UK, due to liver toxicity, in 1982.[4][7][8]
Synthesis
[edit]Clomacran can be synthesized beginning with 2-chloroacridone (1) which is reacted with aGrignard reagentderived from 3-chloro-N,N-dimethylpropylamine (2) to afford the tertiary carbinol (3).[9][10][11][12]Dehydrationby means of acid or simply heat gives the corresponding olefin (4). Catalytic reduction completes the synthesis of clomacran (5).

References
[edit]- ^abc"Clomacran | 5310-55-4".ChemicalBook.Retrieved2023-08-25.
- ^abElks J, Ganellin CR, eds. (1990).Dictionary of Drugs.Boston, MA: Springer US. p. 297.doi:10.1007/978-1-4757-2085-3.ISBN978-1-4757-2087-7.
- ^"Substâncias e remédios sob controle"[Substances and drugs under control](PDF).Jornal do Brasil(in Brazilian Portuguese). 1986-11-05. p. 14.Archived(PDF)from the original on 2023-08-08.Retrieved2023-08-08.
- ^abDixit N, Patel C, Bhavsar M, Patel S, Rawal R, Solanki H (2022-05-02)."Quantitative Structure-activity Relationship (QSAR) study of Liver Toxic Drugs".International Association of Biologicals and Computational Digest.1:63–71.doi:10.56588/iabcd.v1i1.17.eISSN2583-3995.
- ^Anvisa(2023-03-31)."RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial"[Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese).Diário Oficial da União(published 2023-04-04).Archivedfrom the original on 2023-08-03.Retrieved2023-08-03.
- ^Pecknold JC, Ban TA, Lehmann HE, Climan M (June 1975). "Clomacran in the treatment of schizophrenic patients: a comparison of two assessment methods".International Journal of Clinical Pharmacology and Biopharmacy.11(4): 299–303.PMID1099021.
- ^"Clomacran".PubChem.U.S. National Library of Medicine.Retrieved2023-08-25.
- ^Andrews EB, Moore N, eds. (2014).Mann's Pharmacovigilance(1st ed.). Wiley.doi:10.1002/9781118820186.ISBN978-0-470-67104-7.
- ^Zirkle Charles L,U.S. patent 3,131,190(1964 to Smith Kline French Lab).
- ^E Anderson & H Graboyes,U.S. patent 3,781,358(1973 to SmithKline Beecham Corp).
- ^Elvin L Anderson & Harold Graboyes,U.S. patent 3,692,834(1972 to Smith Kline and French Laboratories Ltd, GlaxoSmithKline LLC SmithKline Beecham Corp).
- ^Elvin L Anderson & Harold Graboyes,U.S. patent 3,919,312(1975 to SmithKline Beecham Corp).
