Methane
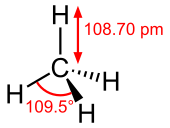
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methane[1] | |||
| Systematic IUPAC name
Carbane (never recommended[1]) | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 1718732 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.739 | ||
| EC Number |
| ||
| 59 | |||
| KEGG | |||
| MeSH | Methane | ||
PubChemCID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1971 | ||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| CH4 | |||
| Molar mass | 16.043g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Odorless | ||
| Density | |||
| Melting point | −182.456 °C (−296.421 °F; 90.694 K)[3] | ||
| Boiling point | −161.5 °C (−258.7 °F; 111.6 K)[3] | ||
| Critical point(T,P) | 190.56 K (−82.59 °C; −116.66 °F), 4.5992 MPa (45.391 atm) | ||
| 22.7 mg/L[4] | |||
| Solubility | Soluble inethanol,diethyl ether,benzene,toluene,methanol,acetoneand insoluble inwater | ||
| logP | 1.09 | ||
| 14 nmol/(Pa·kg) | |||
| Conjugate acid | Methanium | ||
| Conjugate base | Methyl anion | ||
| −17.4×10−6cm3/mol[5] | |||
| Structure | |||
| Td | |||
| Tetrahedralatcarbonatom | |||
| 0D | |||
| Thermochemistry[6] | |||
| 35.7 J/(K·mol) | |||
Std molar
entropy(S⦵298) |
186.3 J/(K·mol) | ||
Std enthalpy of
formation(ΔfH⦵298) |
−74.6 kJ/mol | ||
Gibbs free energy(ΔfG⦵)
|
−50.5 kJ/mol | ||
Std enthalpy of
combustion(ΔcH⦵298) |
−891 kJ/mol | ||
| Hazards[7] | |||
| GHSlabelling: | |||

| |||
| Danger | |||
| H220 | |||
| P210 | |||
| NFPA 704(fire diamond) | |||
| Flash point | −188 °C (−306.4 °F; 85.1 K) | ||
| 537 °C (999 °F; 810 K) | |||
| Explosive limits | 4.4–17% | ||
| Related compounds | |||
Related alkanes
|
|||
Related compounds
|
|||
| Supplementary data page | |||
| Methane (data page) | |||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
Methane(US:/ˈmɛθeɪn/METH-ayn,UK:/ˈmiːθeɪn/MEE-thayn) is achemical compoundwith thechemical formulaCH4(onecarbonatom bonded to fourhydrogenatoms). It is agroup-14 hydride,the simplestalkane,and the main constituent ofnatural gas.The abundance of methane onEarthmakes it an economically attractivefuel,although capturing and storing it is difficult because it is agasatstandard temperature and pressure.
Naturally occurring methane is found both below ground and under theseafloorand is formed by both geological and biological processes. The largestreservoirof methane is under the seafloor in the form ofmethane clathrates.When methane reaches the surface and theatmosphere,it is known asatmospheric methane.[9]
The Earth's atmospheric methane concentrationhas increasedby about 160% since 1750, with the overwhelming percentage caused by human activity.[10]It accounted for 20% of the totalradiative forcingfrom all of the long-lived and globally mixedgreenhouse gases,according to the 2021Intergovernmental Panel on Climate Changereport.[11]Strong, rapid and sustained reductions in methane emissions could limit near-term warming and improve air quality by reducing global surface ozone.[12]
Methane has also been detected on other planets, includingMars,which has implications forastrobiologyresearch.[13]
Properties and bonding
[edit]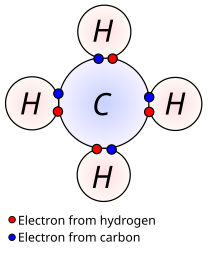
Methane is atetrahedralmolecule with four equivalentC–H bonds.Itselectronic structureis described by four bonding molecular orbitals (MOs) resulting from the overlap of the valence orbitals onCandH.The lowest-energy MO is the result of the overlap of the 2s orbital on carbon with the in-phase combination of the 1s orbitals on the four hydrogen atoms. Above this energy level is a triply degenerate set of MOs that involve overlap of the 2p orbitals on carbon with various linear combinations of the 1s orbitals on hydrogen. The resulting "three-over-one" bonding scheme is consistent with photoelectron spectroscopic measurements.
Methane is an odorless, colourless and transparent gas.[14]It does absorb visible light, especially at the red end of the spectrum, due toovertone bands,but the effect is only noticeable if the light path is very long. This is what givesUranusandNeptunetheir blue or bluish-green colors, as light passes through their atmospheres containing methane and is then scattered back out.[15]
The familiar smell of natural gas as used in homes is achieved by the addition of anodorant,usually blends containingtert-butylthiol,as a safety measure. Methane has a boiling point of −161.5°Cat a pressure of oneatmosphere.[3]As a gas, it isflammableover a range of concentrations (5.4%–17%) in air atstandard pressure.
Solid methane exists in severalmodifications.Presently nine are known.[16]Cooling methane at normal pressure results in the formation of methane I. This substance crystallizes in the cubic system (space groupFm3m). The positions of the hydrogen atoms are not fixed in methane I, i.e. methane molecules may rotate freely. Therefore, it is aplastic crystal.[17]
Chemical reactions
[edit]The primary chemical reactions of methane arecombustion,steam reformingtosyngas,andhalogenation.In general, methane reactions are difficult to control.
Selective oxidation
[edit]Partialoxidationof methane tomethanol(CH3OH), a more convenient, liquid fuel, is challenging because the reaction typically progresses all the way tocarbon dioxideandwatereven with an insufficient supply ofoxygen.Theenzymemethane monooxygenaseproduces methanol from methane, but cannot be used for industrial-scale reactions.[18]Some homogeneouslycatalyzedsystems and heterogeneous systems have been developed, but all have significant drawbacks. These generally operate by generating protected products which are shielded from overoxidation. Examples include theCatalytica system,copperzeolites,and iron zeolites stabilizing thealpha-oxygenactive site.[19]
One group ofbacteriacatalyze methane oxidation withnitriteas theoxidantin the absence ofoxygen,giving rise to the so-calledanaerobic oxidation of methane.[20]
Acid–base reactions
[edit]Like otherhydrocarbons,methane is an extremelyweak acid.ItspKainDMSOis estimated to be 56.[21]It cannot bedeprotonatedin solution, but theconjugate baseis known in forms such asmethyllithium.
A variety ofpositive ionsderived from methane have been observed, mostly as unstable species in low-pressure gas mixtures. These includemetheniumor methyl cationCH+3,methane cationCH+4,andmethaniumor protonated methaneCH+5.Some of these have beendetected in outer space.Methanium can also be produced as diluted solutions from methane withsuperacids.Cationswith higher charge, such asCH2+6andCH3+7,have been studied theoretically and conjectured to be stable.[22]
Despite thestrengthof its C–H bonds, there is intense interest incatalyststhat facilitateC–H bond activationin methane (and other lower numberedalkanes).[23]
Combustion
[edit]
Methane'sheat of combustionis 55.5 MJ/kg.[24]Combustionof methane is a multiple step reaction summarized as follows:
Peters four-step chemistryis a systematically reduced four-step chemistry that explains the burning of methane.
Methane radical reactions
[edit]Given appropriate conditions, methane reacts withhalogenradicalsas follows:
- •X + CH4→ HX + •CH3
- •CH3+ X2→ CH3X + •X
where X is ahalogen:fluorine(F),chlorine(Cl),bromine(Br), oriodine(I). This mechanism for this process is calledfree radical halogenation.It is initiated whenUV lightor some otherradical initiator(likeperoxides) produces a halogenatom.A two-stepchain reactionensues in which the halogen atom abstracts a hydrogen atom from a methane molecule, resulting in the formation of ahydrogen halidemolecule and amethyl radical(•CH3). The methyl radical then reacts with a molecule of the halogen to form a molecule of the halomethane, with a new halogen atom as byproduct.[25]Similar reactions can occur on the halogenated product, leading to replacement of additional hydrogen atoms by halogen atoms withdihalomethane,trihalomethane,and ultimately,tetrahalomethanestructures, depending upon reaction conditions and the halogen-to-methane ratio.
This reaction is commonly used with chlorine to producedichloromethaneandchloroformviachloromethane.Carbon tetrachloridecan be made with excess chlorine.
Uses
[edit]Methane may be transported as a refrigerated liquid (liquefied natural gas, orLNG). While leaks from a refrigerated liquid container are initially heavier than air due to the increased density of the cold gas, the gas at ambient temperature is lighter than air.Gas pipelinesdistribute large amounts of natural gas, of which methane is the principal component.
Fuel
[edit]Methane is used as afuelfor ovens, homes, water heaters, kilns, automobiles,[26][27]turbines, etc.
As the major constituent ofnatural gas,methane is important forelectricity generationby burning it as a fuel in agas turbineorsteam generator.Compared to otherhydrocarbon fuels,methane produces lesscarbon dioxidefor each unit of heat released. At about 891 kJ/mol, methane'sheat of combustionis lower than that of any other hydrocarbon, but the ratio of the heat of combustion (891 kJ/mol) to the molecular mass (16.0 g/mol, of which 12.0 g/mol is carbon) shows that methane, being the simplest hydrocarbon, produces more heat per mass unit (55.7 kJ/g) than other complex hydrocarbons. In many areas with a dense enough population, methane is piped into homes and businesses forheating,cooking, and industrial uses. In this context it is usually known asnatural gas,which is considered to have an energy content of 39megajoulesper cubic meter, or 1,000BTUperstandard cubic foot.Liquefied natural gas(LNG) is predominantly methane (CH4) converted into liquid form for ease of storage or transport.
Rocket propellant
[edit]Refinedliquid methaneas well as LNG isused asarocket fuel,[28]when combined withliquid oxygen,as in theTQ-12,BE-4,Raptor,andYF-215engines.[29]Due to the similarities between methane and LNG such engines are commonly grouped together under the termmethalox.
As aliquid rocketpropellant, a methane/liquid oxygencombination offers the advantage overkerosene/liquid oxygencombination, or kerolox, of producing small exhaust molecules, reducing coking or deposition ofsooton engine components. Methane is easier to store than hydrogen due to its higher boiling point and density, as well as its lack ofhydrogen embrittlement.[30][31]The lowermolecular weightof the exhaust also increases the fraction of the heat energy which is in the form of kinetic energy available for propulsion, increasing thespecific impulseof the rocket. Compared toliquid hydrogen,thespecific energyof methane is lower but this disadvantage is offset by methane's greater density and temperature range, allowing for smaller and lighter tankage for a given fuel mass. Liquid methane has a temperature range (91–112 K) nearly compatible withliquid oxygen(54–90 K). The fuel currently sees use in operational launch vehicles such asZhuque-2andVulcanas well as in-development launchers such asStarship,Neutron,andTerran R.[32]
Chemical feedstock
[edit]Natural gas,which is mostly composed of methane, is used to produce hydrogen gas on an industrial scale.Steam methane reforming(SMR), or simply known as steam reforming, is the standard industrial method of producing commercial bulk hydrogen gas. More than 50 million metric tons are produced annually worldwide (2013), principally from the SMR of natural gas.[33]Much of this hydrogen is used inpetroleumrefineries,in the production of chemicals and in food processing. Very large quantities of hydrogen are used in theindustrial synthesis of ammonia.
At high temperatures (700–1100 °C) and in the presence of ametal-basedcatalyst(nickel), steam reacts with methane to yield a mixture ofCOandH2,known as "water gas" or "syngas":
- CH4+ H2O ⇌ CO + 3 H2
This reaction is stronglyendothermic(consumes heat,ΔHr=206 kJ/mol). Additional hydrogen is obtained by the reaction ofCOwith water via thewater-gas shift reaction:
- CO + H2O ⇌ CO2+ H2
This reaction is mildlyexothermic(produces heat,ΔHr=−41 kJ/mol).
Methane is also subjected to free-radicalchlorinationin the production of chloromethanes, althoughmethanolis a more typical precursor.[34]
Hydrogen can also be produced via the direct decomposition of methane, also known as methanepyrolysis,which, unlike steam reforming, produces nogreenhouse gases(GHG). The heat needed for the reaction can also be GHG emission free, e.g. from concentrated sunlight, renewable electricity, or burning some of the produced hydrogen. If the methane is frombiogasthen the process can be acarbon sink.Temperatures in excess of 1200 °C are required to break the bonds of methane to produce Hydrogen gas and solid carbon. However, through the use of a suitable catalyst the reaction temperature can be reduced to between 600 °C - 1000 °C depending on the chosen catalyst.[35]The reaction is moderately endothermic as shown in the reaction equation below.[36]
- CH4(g) → C(s) + 2 H2(g)
- (ΔH° =74.8kJ/mol)
Refrigerant
[edit]As arefrigerant,methane has theASHRAEdesignationR-50.
Generation
[edit]
Methane can be generated through geological, biological or industrial routes.
Geological routes
[edit]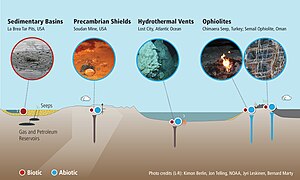
The two main routes for geological methane generation are (i) organic (thermally generated, or thermogenic) and (ii) inorganic (abiotic).[13]Thermogenic methane occurs due to the breakup of organic matter at elevated temperatures and pressures in deep sedimentarystrata.Most methane in sedimentary basins is thermogenic; therefore, thermogenic methane is the most important source of natural gas. Thermogenic methane components are typically considered to be relic (from an earlier time). Generally, formation of thermogenic methane (at depth) can occur through organic matter breakup, or organic synthesis. Both ways can involve microorganisms (methanogenesis), but may also occur inorganically. The processes involved can also consume methane, with and without microorganisms.
The more important source of methane at depth (crystalline bedrock) is abiotic. Abiotic means that methane is created from inorganic compounds, without biological activity, either through magmatic processes[example needed]or via water-rock reactions that occur at low temperatures and pressures, likeserpentinization.[37][38]
Biological routes
[edit]Most of Earth's methane isbiogenicand is produced bymethanogenesis,[39][40]a form of anaerobic respiration only known to be conducted by some members of the domainArchaea.[41]Methanogens occur inlandfillsandsoils,[42]ruminants(for example,cattle),[43]the guts of termites, and theanoxicsediments below the seafloor and the bottom of lakes.
This multistep process is used by these microorganisms for energy. The net reaction of methanogenesis is:
- CO2+ 4 H2→ CH4+ 2 H2O
The final step in the process is catalyzed by the enzymemethyl coenzyme M reductase(MCR).[44]

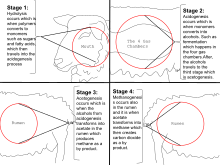
Wetlands
[edit]Wetlands are the largest natural sources of methane to the atmosphere,[45]accounting for approximately 20 - 30% of atmospheric methane.[46]Climate change is increasing the amount of methane released from wetlands due to increased temperatures and altered rainfall patterns. This phenomeon is calledwetland methane feedback.[47]
Ricecultivation generates as much as 12% of total global methane emissions due to the long-term flooding of rice fields.[48]
Ruminants
[edit]Ruminants, such as cattle, belch methane, accounting for about 22% of the U.S. annual methane emissions to the atmosphere.[49]One study reported that the livestock sector in general (primarily cattle, chickens, and pigs) produces 37% of all human-induced methane.[50]A 2013 study estimated that livestock accounted for 44% of human-induced methane and about 15% of human-induced greenhouse gas emissions.[51]Many efforts are underway to reduce livestock methane production, such as medical treatments and dietary adjustments,[52][53]and to trap the gas to use its combustion energy.[54]
Seafloor sediments
[edit]Most of the subseafloor isanoxicbecause oxygen is removed byaerobicmicroorganisms within the first few centimeters of thesediment.Below the oxygen-replete seafloor, methanogens produce methane that is either used by other organisms or becomes trapped ingas hydrates.[41]These other organisms that utilize methane for energy are known asmethanotrophs('methane-eating'), and are the main reason why little methane generated at depth reaches the sea surface.[41]Consortia of Archaea and Bacteria have been found to oxidize methane viaanaerobic oxidation of methane(AOM); the organisms responsible for this are anaerobicmethanotrophicArchaea (ANME) andsulfate-reducing bacteria(SRB).[55]
Industrial routes
[edit]
Given its cheap abundance in natural gas, there is little incentive to produce methane industrially. Methane can be produced byhydrogenatingcarbon dioxide through theSabatier process.Methane is also a side product of the hydrogenation of carbon monoxide in theFischer–Tropsch process,which is practiced on a large scale to produce longer-chain molecules than methane.
An example of large-scale coal-to-methane gasification is theGreat Plains Synfuelsplant, started in 1984 inBeulah, North Dakotaas a way to develop abundant local resources of low-gradelignite,a resource that is otherwise difficult to transport for its weight,ashcontent, low calorific value and propensity tospontaneous combustionduring storage and transport. A number of similar plants exist around the world, although mostly these plants are targeted towards the production of long chain alkanes for use asgasoline,diesel,or feedstock to other processes.
Power to methaneis a technology that useselectrical powerto produce hydrogen from water byelectrolysisand uses theSabatier reactionto combine hydrogen withcarbon dioxideto produce methane.
Laboratory synthesis
[edit]Methane can be produced byprotonationofmethyl lithiumor a methylGrignard reagentsuch asmethylmagnesium chloride.It can also be made from anhydroussodium acetateand drysodium hydroxide,mixed and heated above 300 °C (withsodium carbonateas byproduct).[citation needed]In practice, a requirement for pure methane can easily be fulfilled by steel gas bottle from standard gas suppliers.
Occurrence
[edit]Methane was discovered and isolated byAlessandro Voltabetween 1776 and 1778 when studyingmarsh gasfromLake Maggiore.It is the major component of natural gas, about 87% by volume. The major source of methane is extraction from geological deposits known asnatural gas fields,withcoal seam gasextraction becoming a major source (seecoal bed methane extraction,a method for extracting methane from acoaldeposit, whileenhanced coal bed methane recoveryis a method of recovering methane from non-mineable coal seams). It is associated with otherhydrocarbonfuels, and sometimes accompanied byheliumandnitrogen.Methane is produced at shallow levels (low pressure) byanaerobicdecayoforganic matterand reworked methane from deep under the Earth's surface. In general, thesedimentsthat generate natural gas are buried deeper and at higher temperatures than those that containoil.
Methane is generally transported in bulk bypipelinein its natural gas form, or by LNG carriers in its liquefied form; few countries transport it by truck.
Atmospheric methane and climate change
[edit]
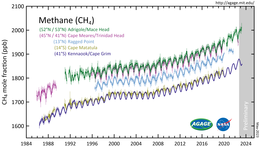
Methane is an importantgreenhouse gas,responsible for around 30% of the rise in global temperatures since the industrial revolution.[56]
Methane has aglobal warming potential(GWP) of 29.8 ± 11 compared toCO2(potential of 1) over a 100-year period, and 82.5 ± 25.8 over a 20-year period.[57]This means that, for example, aleakof one tonne of methane is equivalent to emitting 82.5 tonnes of carbon dioxide. Burning methane and producing carbon dioxide also reduces the greenhouse gas impact compared to simply venting methane to the atmosphere.

As methane is gradually converted into carbon dioxide (and water) in the atmosphere, these values include the climate forcing from the carbon dioxide produced from methane over these timescales.
Annual global methane emissions are currently approximately 580 Mt,[58]40% of which is from natural sources and the remaining 60% originating from human activity, known as anthropogenic emissions. The largest anthropogenic source isagriculture,responsible for around one quarter of emissions, closely followed by theenergy sector,which includes emissions from coal, oil, natural gas and biofuels.[59]
Historic methane concentrationsin the world's atmosphere have ranged between 300 and 400 nmol/mol during glacial periods commonly known asice ages,and between 600 and 700 nmol/mol during the warminterglacialperiods. A 2012 NASA website said the oceans were a potential important source of Arctic methane,[60]but more recent studies associate increasing methane levels as caused by human activity.[10]
Global monitoring of atmospheric methane concentrations began in the 1980s.[10]The Earth's atmospheric methane concentration has increased 160% since preindustrial levels in the mid-18th century.[10]In 2013, atmospheric methane accounted for 20% of the totalradiative forcingfrom all of the long-lived and globally mixed greenhouse gases.[61]Between 2011 and 2019 the annual average increase of methane in the atmosphere was 1866 ppb.[11]From 2015 to 2019 sharp rises in levels of atmospheric methane were recorded.[62][63]
In 2019, the atmospheric methane concentration was higher than at any time in the last 800,000 years. As stated in theAR6of theIPCC,"Since 1750, increases inCO2(47%) andCH4(156%) concentrations far exceed, and increases inN2O(23%) are similar to, the natural multi-millennial changes between glacial and interglacial periods over at least the past 800,000 years (very high confidence) ".[11][a][64]
In February 2020, it was reported thatfugitive emissionsandgas ventingfrom thefossil fuel industrymay have been significantly underestimated.[65] [66]The largest annual increase occurred in 2021 with the overwhelming percentage caused by human activity.[10]
Climate change can increase atmospheric methane levels by increasing methane production in natural ecosystems, forming aclimate change feedback.[41][67]Another explanation for the rise in methane emissions could be a slowdown of the chemical reaction that removes methane from the atmosphere.[68]
Over 100 countries have signed theGlobal Methane Pledge,launched in 2021, promising to cut their methane emissions by 30% by 2030.[69]This could avoid 0.2˚C of warming globally by 2050, although there have been calls for higher commitments in order to reach this target.[70]TheInternational Energy Agency's 2022 report states "the most cost-effective opportunities for methane abatement are in the energy sector, especially in oil and gas operations".[71]
Clathrates
[edit]Methane clathrates(also known as methane hydrates) are solid cages of water molecules that trap single molecules of methane. Significant reservoirs of methane clathrates have been found in arctic permafrost and alongcontinental marginsbeneath theocean floorwithin thegas clathrate stability zone,located at high pressures (1 to 100 MPa; lower end requires lower temperature) and low temperatures (< 15 °C; upper end requires higher pressure).[72]Methane clathrates can form from biogenic methane, thermogenic methane, or a mix of the two. These deposits are both a potential source of methane fuel as well as a potential contributor to global warming.[73][74]The global mass of carbon stored in gas clathrates is still uncertain and has been estimated as high as 12,500Gtcarbon and as low as 500 Gt carbon.[47]The estimate has declined over time with a most recent estimate of ≈1800 Gt carbon.[75]A large part of this uncertainty is due to our knowledge gap in sources and sinks of methane and the distribution of methane clathrates at the global scale. For example, a source of methane was discovered relatively recently in anultraslow spreading ridgein the Arctic.[46]Some climate models suggest that today's methane emission regime from the ocean floor is potentially similar to that during the period of thePaleocene–Eocene Thermal Maximum(PETM) around 55.5 million years ago, although there are no data indicating that methane from clathrate dissociation currently reaches the atmosphere.[75]Arctic methane releasefrompermafrostand seafloor methane clathrates is a potential consequence and further cause ofglobal warming;this is known as theclathrate gun hypothesis.[76][77][78][79]Data from 2016 indicate that Arctic permafrost thaws faster than predicted.[80]
Public safety and the environment
[edit]
Methane "degrades air quality and adversely impacts human health, agricultural yields, and ecosystem productivity".[81]
Methane is extremely flammable and may formexplosivemixtures with air. Methane gas explosions are responsible for many deadly mining disasters.[82]A methane gas explosion was the cause of theUpper Big Branch coal mine disasterinWest Virginiaon April 5, 2010, killing 29.[83]Natural gas accidental releasehas also been a major focus in the field ofsafety engineering,due to past accidental releases that concluded in the formation ofjet firedisasters.[84][85]
The 2015–2016methane gas leak in Aliso Canyon, Californiawas considered to be the worst in terms of its environmental effect in American history.[86][87][88]It was also described as more damaging to the environment thanDeepwater Horizon's leak in the Gulf of Mexico.[89]
In May 2023The Guardianpublished a report, blamingTurkmenistanto be the worst in the world for methanesuper emitting.The data collected by Kayrros researchers indicate, that two large Turkmen fossil fuel fields leaked 2.6 million and 1.8 millionmetric tonnesof methane in 2022 alone, pumping theCO2equivalent of 366 million tonnes into the atmosphere, surpassing the annual CO2emissions of theUnited Kingdom.[90]
Methane is also anasphyxiantif the oxygen concentration is reduced to below about 16% by displacement, as most people cantolerate a reduction from 21% to 16% without ill effects.The concentration of methane at which asphyxiation risk becomes significant is much higher than the 5–15% concentration in a flammable or explosive mixture. Methane off-gas can penetrate the interiors of buildings nearlandfillsand expose occupants to significant levels of methane. Some buildings have specially engineered recovery systems below their basements to actively capture this gas and vent it away from the building.
Extraterrestrial methane
[edit]Interstellar medium
[edit]This sectionis missing informationabout where extraterrestrial abiotic methane comes from (Big Bang? supernova? mineral deposits reacting?).(June 2024) |
Methane is abundant in many parts of the Solar System and potentially could be harvested on the surface of another Solar System body (in particular, usingmethane production from local materialsfound onMars[91]orTitan), providing fuel for a return journey.[28][92]
Mars
[edit]Methane has been detected on all planets of theSolar Systemand most of the larger moons.[citation needed]With the possible exception ofMars,it is believed to have come fromabioticprocesses.[93][94]

TheCuriosityroverhas documented seasonal fluctuations ofatmospheric methanelevels on Mars. These fluctuations peaked at the end of the Martian summer at 0.6 parts per billion.[95][96][97][98][99][100][101][102]
Methane has been proposed as a possiblerocket propellanton futureMars missionsdue in part to the possibility of synthesizing it on the planet byin situ resource utilization.[103]An adaptation of theSabatier methanation reactionmay be used with a mixed catalyst bed and areverse water-gas shiftin a single reactor to produce methane andoxygenfrom the raw materials available on Mars, utilizing water from theMartian subsoilandcarbon dioxidein theMartian atmosphere.[91]
Methane could be produced by a non-biological process calledserpentinization[b]involving water, carbon dioxide, and the mineralolivine,which is known to be common on Mars.[104]
Titan
[edit]
Methane has been detected in vast abundance onTitan,the largest moon ofSaturn,it comprises a significant portion of itsatmosphereand also exists in a liquid form on its surface, where it comprises the majority of the liquid in Titan's vastlakesof hydrocarbons; thesecond largestof which is believed to be almost pure methane in composition.[105]
The presence of stable lakes of liquid methane on Titan, as well as the surface of Titan being highly chemically active and rich in organic compounds, has led scientists to consider the possibility oflifeexisting within Titan's lakes, using methane as a solvent in the place of water for Earth-based life[106]and using hydrogen in the atmosphere to derive energy withacetylene,in much the same way that Earth-based life usesglucose.[107]
History
[edit]
Methane was first scientifically identified in November 1776 byItalianphysicistAlessandro Voltain the marshes ofLake MaggiorestraddlingItalyandSwitzerland.Volta was inspired to search for the substance after reading a paper written byBenjamin Franklinabout "flammable air".[108]Volta collected the gas rising from the marsh, and by 1778 had isolated pure methane.[109]He also demonstrated that the gas could be ignited with an electric spark.[109]
Following theFelling mine disasterof 1812 in which 92 men perished, SirHumphry Davyestablished that the fearedfiredampwas in fact largely methane.[110]
The name "methane" was coined in 1866 by the German chemistAugust Wilhelm von Hofmann.[111][112]The name was derived frommethanol.
Etymology
[edit]Etymologically, the wordmethaneis coined from the chemical suffix "-ane",which denotes substances belonging to the alkane family; and the wordmethyl,which is derived from the GermanMethyl(1840) or directly from the Frenchméthyle,which is a back-formation from the Frenchméthylène(corresponding to English "methylene" ), the root of which was coined byJean-Baptiste DumasandEugène Péligotin 1834 from the Greekμέθυmethy(wine) (related to English "mead" ) andὕληhyle(meaning "wood" ). The radical is named after this because it was first detected inmethanol,an alcohol first isolated by distillation of wood. The chemical suffix-aneis from the coordinating chemical suffix-inewhich is from Latin feminine suffix-inawhich is applied to represent abstracts. The coordination of "-ane", "-ene", "-one", etc. was proposed in 1866 by German chemistAugust Wilhelm von Hofmann.[113]
Abbreviations
[edit]The abbreviationCH4-C can mean the mass of carbon contained in a mass of methane, and the mass of methane is always 1.33 times the mass ofCH4-C.[114][115]CH4-C can also mean the methane-carbon ratio, which is 1.33 by mass.[116]Methane at scales of the atmosphere is commonly measured in teragrams (TgCH4) or millions of metric tons (MMTCH4), which mean the same thing.[117]Other standard units are also used, such as nanomole (nmol, one billionth of a mole),mole(mol),kilogram,andgram.
See also
[edit]- 2007 Zasyadko mine disaster
- Abiogenic petroleum origin
- Aerobic methane production
- Anaerobic digestion
- Anaerobic respiration
- Arctic methane emissions
- Atmospheric methane
- Biogas
- Coal Oil Point seep field
- Energy density
- Fugitive gas emissions
- Global Methane Initiative
- Thomas Gold
- Halomethane,halogenated methane derivatives.
- Hydrogen Cycle
- Industrial gas
- Lake Kivu(more general:limnic eruption)
- List of straight-chain alkanes
- Methanation
- Methane emissions
- Methane on Mars:
- Methanogen,archaeathat produce methane.
- Methanogenesis,microbesthat produce methane.
- Methanotroph,bacteriathat grow with methane.
- Methyl group,a functional group related to methane.
Explanatory notes
[edit]- ^In 2013Intergovernmental Panel on Climate Change(IPCC) scientists warned atmospheric concentrations of methane had "exceeded the pre-industrial levels by about 150% which represented" levels unprecedented in at least the last 800,000 years. "
- ^There are manyserpentinizationreactions.Olivineis asolid solutionbetweenforsteriteandfayalitewhose general formula is(Fe,Mg)2SiO4.The reaction producing methane from olivine can be written as:Forsterite + Fayalite + Water + Carbonic acid → Serpentine + Magnetite + Methane,or (in balanced form):
- 18 Mg2SiO4+ 6 Fe2SiO4+ 26 H2O + CO2→ 12 Mg3Si2O5(OH)4+ 4 Fe3O4+ CH4
Citations
[edit]- ^ab"General Principles, Rules, and Conventions".Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013 (Blue Book).Cambridge:The Royal Society of Chemistry.2014. P-12.1.doi:10.1039/9781849733069-00001.ISBN978-0-85404-182-4.
Methane is a retained name (see P-12.3) that is preferred to the systematic name 'carbane', a name never recommended to replace methane, but used to derive the names 'carbene' and 'carbyne' for the radicals H2C2•and HC3•,respectively.
- ^"Gas Encyclopedia".Archivedfrom the original on December 26, 2018.RetrievedNovember 7,2013.
- ^abcdHaynes,p. 3.344
- ^Haynes,p. 5.156
- ^Haynes,p. 3.578
- ^Haynes,pp. 5.26, 5.67
- ^"Safety Datasheet, Material Name: Methane"(PDF).US: Metheson Tri-Gas Incorporated. December 4, 2009. Archived fromthe original(PDF)on June 4, 2012.RetrievedDecember 4,2011.
- ^NOAA Office of Response and Restoration, US GOV."METHANE".noaa.gov.Archivedfrom the original on January 9, 2019.RetrievedMarch 20,2015.
- ^Khalil, M. A. K. (1999). "Non-Co2 Greenhouse Gases in the Atmosphere".Annual Review of Energy and the Environment.24:645–661.doi:10.1146/annurev.energy.24.1.645.
- ^abcdeGlobal Methane Assessment(PDF).United Nations Environment Programme and Climate and Clean Air Coalition(Report). Nairobi. 2022. p. 12.RetrievedMarch 15,2023.
- ^abc"Climate Change 2021. The Physical Science Basis. Summary for Policymakers. Working Group I contribution to the WGI Sixth Assessment Report of the Intergovernmental Panel on Climate Change".IPCC.The Intergovernmental Panel on Climate Change. Archived fromthe originalon August 22, 2021.RetrievedAugust 22,2021.
- ^IPCC, 2023: Summary for Policymakers.In: Climate Change 2023: Synthesis Report. A Report of the Intergovernmental Panel on Climate Change. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, H. Lee and J. Romero (eds.)]. IPCC, Geneva, Switzerland, page 26, section C.2.3
- ^abEtiope, Giuseppe; Lollar, Barbara Sherwood (2013). "Abiotic Methane on Earth".Reviews of Geophysics.51(2): 276–299.Bibcode:2013RvGeo..51..276E.doi:10.1002/rog.20011.S2CID56457317.
- ^Hensher, David A.; Button, Kenneth J. (2003).Handbook of transport and the environment.Emerald Group Publishing. p. 168.ISBN978-0-08-044103-0.Archivedfrom the original on March 19, 2015.RetrievedFebruary 22,2016.
- ^P.G.J Irwin; et al. (January 12, 2022)."Hazy Blue Worlds: A Holistic Aerosol Model for Uranus and Neptune, Including Dark Spots".Journal of Geophysical Research: Planets.127(6): e2022JE007189.arXiv:2201.04516.Bibcode:2022JGRE..12707189I.doi:10.1029/2022JE007189.PMC9286428.PMID35865671.S2CID245877540.
- ^Bini, R.; Pratesi, G. (1997). "High-pressure infrared study of solid methane: Phase diagram up to 30 GPa".Physical Review B.55(22): 14800–14809.Bibcode:1997PhRvB..5514800B.doi:10.1103/physrevb.55.14800.
- ^Wendelin Himmelheber."Crystal structures".Archivedfrom the original on February 12, 2020.RetrievedDecember 10,2019.
- ^Baik, Mu-Hyun; Newcomb, Martin; Friesner, Richard A.; Lippard, Stephen J. (2003). "Mechanistic Studies on the Hydroxylation of Methane by Methane Monooxygenase".Chemical Reviews.103(6): 2385–419.doi:10.1021/cr950244f.PMID12797835.
- ^Snyder, Benjamin E. R.; Bols, Max L.; Schoonheydt, Robert A.; Sels, Bert F.; Solomon, Edward I. (December 19, 2017)."Iron and Copper Active Sites in Zeolites and Their Correlation to Metalloenzymes".Chemical Reviews.118(5): 2718–2768.doi:10.1021/acs.chemrev.7b00344.PMID29256242.
- ^ Reimann, Joachim; Jetten, Mike S.M.; Keltjens, Jan T. (2015). "Metal Enzymes in" Impossible "Microorganisms Catalyzing the Anaerobic Oxidation of Ammonium and Methane". In Peter M.H. Kroneck and Martha E. Sosa Torres (ed.).Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases.Metal Ions in Life Sciences. Vol. 15. Springer. pp. 257–313.doi:10.1007/978-3-319-12415-5_7.ISBN978-3-319-12414-8.PMID25707470.
- ^Bordwell, Frederick G. (1988). "Equilibrium acidities in dimethyl sulfoxide solution".Accounts of Chemical Research.21(12): 456–463.doi:10.1021/ar00156a004.S2CID26624076.
- ^Rasul, G.; Surya Prakash, G.K.; Olah, G.A. (2011). "Comparative study of the hypercoordinate carbonium ions and their boron analogs: A challenge for spectroscopists".Chemical Physics Letters.517(1): 1–8.Bibcode:2011CPL...517....1R.doi:10.1016/j.cplett.2011.10.020.
- ^Bernskoetter, W. H.; Schauer, C. K.; Goldberg, K. I.; Brookhart, M. (2009). "Characterization of a Rhodium(I) σ-Methane Complex in Solution".Science.326(5952): 553–556.Bibcode:2009Sci...326..553B.doi:10.1126/science.1177485.PMID19900892.S2CID5597392.
- ^Energy Content of some Combustibles (in MJ/kg)ArchivedJanuary 9, 2014, at theWayback Machine.People.hofstra.edu. Retrieved on March 30, 2014.
- ^March, Jerry (1968).Advance Organic Chemistry: Reactions, Mechanisms and Structure.New York: McGraw-Hill Book Company. pp. 533–534.
- ^"Lumber Company Locates Kilns at Landfill to Use Methane – Energy Manager Today".Energy Manager Today.September 23, 2015.Archivedfrom the original on July 9, 2019.RetrievedMarch 11,2016.
- ^Cornell, Clayton B. (April 29, 2008)."Natural Gas Cars: CNG Fuel Almost Free in Some Parts of the Country".Archived fromthe originalon January 20, 2019.RetrievedJuly 25,2009.
Compressed natural gas is touted as the 'cleanest burning' alternative fuel available, since the simplicity of the methane molecule reduces tailpipe emissions of different pollutants by 35 to 97%. Not quite as dramatic is the reduction in net greenhouse-gas emissions, which is about the same as corn-grain ethanol at about a 20% reduction over gasoline
- ^ab Thunnissen, Daniel P.; Guernsey, C. S.; Baker, R. S.; Miyake, R. N. (2004)."Advanced Space Storable Propellants for Outer Planet Exploration"(PDF).American Institute of Aeronautics and Astronautics(4–0799): 28. Archived fromthe original(PDF)on March 10, 2016.
- ^"Blue Origin BE-4 Engine".Archivedfrom the original on October 1, 2021.RetrievedJune 14,2019.
We chose LNG because it is highly efficient, low cost and widely available. Unlike kerosene, LNG can be used to self-pressurize its tank. Known as autogenous repressurization, this eliminates the need for costly and complex systems that draw on Earth's scarce helium reserves. LNG also possesses clean combustion characteristics even at low throttle, simplifying engine reuse compared to kerosene fuels.
- ^"SpaceX propulsion chief elevates crowd in Santa Barbara".Pacific Business Times. February 19, 2014.RetrievedFebruary 22,2014.
- ^Belluscio, Alejandro G. (March 7, 2014)."SpaceX advances drive for Mars rocket via Raptor power".NASAspaceflight.com.RetrievedMarch 7,2014.
- ^"China beats rivals to successfully launch first methane-liquid rocket".Reuters.July 12, 2023.
- ^Report of the Hydrogen Production Expert Panel: A Subcommittee of the Hydrogen & Fuel Cell Technical Advisory CommitteeArchivedFebruary 14, 2020, at theWayback Machine.United States Department of Energy (May 2013).
- ^Rossberg, M.et al.(2006) "Chlorinated Hydrocarbons" inUllmann's Encyclopedia of Industrial Chemistry,Wiley-VCH, Weinheim.doi:10.1002/14356007.a06_233.pub2.
- ^Lumbers, Brock (2022)."Mathematical modelling and simulation of the thermo-catalytic decomposition of methane for economically improved hydrogen production".International Journal of Hydrogen Energy.47(7): 4265–4283.Bibcode:2022IJHE...47.4265L.doi:10.1016/j.ijhydene.2021.11.057.S2CID244814932.RetrievedJune 15,2022.
- ^Lumbers, Brock (2022)."Low-emission hydrogen production via the thermo-catalytic decomposition of methane for the decarbonisation of iron ore mines in Western Australia".International Journal of Hydrogen Energy.47(37): 16347–16361.Bibcode:2022IJHE...4716347L.doi:10.1016/j.ijhydene.2022.03.124.S2CID248018294.RetrievedJuly 10,2022.
- ^Kietäväinen and Purkamo (2015)."The origin, source, and cycling of methane in deep crystalline rock biosphere".Front. Microbiol.6:725.doi:10.3389/fmicb.2015.00725.PMC4505394.PMID26236303.
- ^Cramer and Franke (2005)."Indications for an active petroleum system in the Laptev Sea, NE Siberia".Journal of Petroleum Geology.28(4): 369–384.Bibcode:2005JPetG..28..369C.doi:10.1111/j.1747-5457.2005.tb00088.x.S2CID129445357.Archivedfrom the original on October 1, 2021.RetrievedMay 23,2017.
- ^Lessner, Daniel J. (Dec 2009) Methanogenesis Biochemistry. In: eLS. John Wiley & Sons Ltd, Chichester.http://www.els.netArchivedMay 13, 2011, at theWayback Machine
- ^Thiel, Volker (2018), "Methane Carbon Cycling in the Past: Insights from Hydrocarbon and Lipid Biomarkers", in Wilkes, Heinz (ed.),Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate,Handbook of Hydrocarbon and Lipid Microbiology, Springer International Publishing, pp. 1–30,doi:10.1007/978-3-319-54529-5_6-1,ISBN9783319545295,S2CID105761461
- ^abcdDean, Joshua F.; Middelburg, Jack J.; Röckmann, Thomas; Aerts, Rien; Blauw, Luke G.; Egger, Matthias; Jetten, Mike S. M.; de Jong, Anniek E. E.; Meisel, Ove H. (2018)."Methane Feedbacks to the Global Climate System in a Warmer World".Reviews of Geophysics.56(1): 207–250.Bibcode:2018RvGeo..56..207D.doi:10.1002/2017RG000559.hdl:1874/366386.
- ^Serrano-Silva, N.; Sarria-Guzman, Y.; Dendooven, L.; Luna-Guido, M. (2014). "Methanogenesis and methanotrophy in soil: a review".Pedosphere.24(3): 291–307.Bibcode:2014Pedos..24..291S.doi:10.1016/s1002-0160(14)60016-3.
- ^Sirohi, S. K.; Pandey, Neha; Singh, B.; Puniya, A. K. (September 1, 2010)."Rumen methanogens: a review".Indian Journal of Microbiology.50(3): 253–262.doi:10.1007/s12088-010-0061-6.PMC3450062.PMID23100838.
- ^Lyu, Zhe; Shao, Nana; Akinyemi, Taiwo; Whitman, William B. (2018)."Methanogenesis".Current Biology.28(13): R727–R732.Bibcode:2018CBio...28.R727L.doi:10.1016/j.cub.2018.05.021.PMID29990451.
- ^Tandon, Ayesha (March 20, 2023)."'Exceptional' surge in methane emissions from wetlands worries scientists ".Carbon Brief.RetrievedSeptember 18,2023.
- ^ab"New source of methane discovered in the Arctic Ocean".phys.org.May 1, 2015.Archivedfrom the original on April 10, 2019.RetrievedApril 10,2019.
- ^abBoswell, Ray; Collett, Timothy S. (2011). "Current perspectives on gas hydrate resources".Energy Environ. Sci.4(4): 1206–1215.doi:10.1039/c0ee00203h.
- ^Global Environment Facility (December 7, 2019)."We can grow more climate-friendly rice".Climate Home News.RetrievedSeptember 18,2023.
- ^"Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2014".2016.Archivedfrom the original on April 12, 2019.RetrievedApril 11,2019.[page needed]
- ^FAO (2006).Livestock's Long Shadow–Environmental Issues and Options.Rome, Italy: Food and Agriculture Organization of the United Nations (FAO).Archivedfrom the original on July 26, 2008.RetrievedOctober 27,2009.
- ^Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A. & Tempio, G. (2013)."Tackling Climate Change Through Livestock".Rome: Food and Agriculture Organization of the United Nations (FAO). Archived fromthe originalon July 19, 2016.RetrievedJuly 15,2016.
- ^Roach, John (May 13, 2002)."New Zealand Tries to Cap Gaseous Sheep Burps".National Geographic.Archived fromthe originalon June 4, 2011.RetrievedMarch 2,2011.
- ^Roque, Breanna M.; Venegas, Marielena; Kinley, Robert D.; Nys, Rocky de; Duarte, Toni L.; Yang, Xiang; Kebreab, Ermias (March 17, 2021)."Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers".PLOS ONE.16(3): e0247820.Bibcode:2021PLoSO..1647820R.doi:10.1371/journal.pone.0247820.ISSN1932-6203.PMC7968649.PMID33730064.
- ^Silverman, Jacob (July 16, 2007)."Do cows pollute as much as cars?".HowStuffWorks.com.Archivedfrom the original on November 4, 2012.RetrievedNovember 7,2012.
- ^Knittel, K.; Wegener, G.; Boetius, A. (2019), McGenity, Terry J. (ed.), "Anaerobic Methane Oxidizers",Microbial Communities Utilizing Hydrocarbons and Lipids: Members, Metagenomics and Ecophysiology,Handbook of Hydrocarbon and Lipid Microbiology, Springer International Publishing, pp. 1–21,doi:10.1007/978-3-319-60063-5_7-1,ISBN9783319600635
- ^"Methane and climate change – Global Methane Tracker 2022 – Analysis".IEA.2022.RetrievedSeptember 18,2023.
- ^Forster, P.; Storelvmo, T.; Armour, K.; Collins, W.; Dufresne, J.-L.; Frame, D.; Lunt, D.J.; Mauritsen, T.; Palmer, M.D.; Watanabe, M.; Wild, M.; Zhang, H. (2021)."The Earth's Energy Budget, Climate Feedbacks, and Climate Sensitivity".Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change.Cambridge, United Kingdom and New York, NY, US: Cambridge University Press. pp. 923–1054.
- ^"Global Methane Budget 2020".www.globalcarbonproject.org.RetrievedSeptember 18,2023.
- ^"Methane and climate change – Global Methane Tracker 2022 – Analysis".IEA.RetrievedSeptember 18,2023.
- ^"Study Finds Surprising Arctic Methane Emission Source".NASA.April 22, 2012.Archivedfrom the original on August 4, 2014.RetrievedMarch 30,2014.
- ^IPCC."Anthropogenic and Natural Radiative Forcing",Climate Change 2013 – The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change.,Cambridge University Press, pp. 659–740, 2013,doi:10.1017/cbo9781107415324.018,ISBN9781107057999,retrievedSeptember 18,2023
- ^Nisbet, E.G. (February 5, 2019)."Very Strong Atmospheric Methane Growth in the 4 Years 2014–2017: Implications for the Paris Agreement".Global Biogeochemical Cycles.33(3): 318–342.Bibcode:2019GBioC..33..318N.doi:10.1029/2018GB006009.
- ^McKie, Robin (February 2, 2017)."Sharp rise in methane levels threatens world climate targets".The Observer.ISSN0029-7712.Archivedfrom the original on July 30, 2019.RetrievedJuly 14,2019.
- ^IPCC(2013). Stocker, T. F.; Qin, D.; Plattner, G.-K.; Tignor, M.; et al. (eds.).Climate Change 2013: The Physical Science Basis(PDF)(Report). Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change.
- ^Hmiel, Benjamin; Petrenko, V. V.; Dyonisius, M. N.; Buizert, C.; Smith, A. M.; Place, P. F.; Harth, C.; Beaudette, R.; Hua, Q.; Yang, B.; Vimont, I.; Michel, S. E.; Severinghaus, J. P.; Etheridge, D.; Bromley, T.; Schmitt, J.; Faïn, X.; Weiss, R. F.; Dlugokencky, E. (February 2020)."Preindustrial 14CH4 indicates greater anthropogenic fossil CH4 emissions".Nature.578(7795): 409–412.Bibcode:2020Natur.578..409H.doi:10.1038/s41586-020-1991-8.ISSN1476-4687.PMID32076219.S2CID211194542.RetrievedMarch 15,2023.
- ^Harvey, Chelsea (February 21, 2020)."Methane Emissions from Oil and Gas May Be Significantly Underestimated; Estimates of methane coming from natural sources have been too high, shifting the burden to human activities".E&E NewsviaScientific American.Archived fromthe originalon February 24, 2020.
- ^Carrington, Damian (July 21, 2020)First active leak of sea-bed methane discovered in AntarcticaArchivedJuly 22, 2020, at theWayback Machine,The Guardian
- ^Ravilious, Kate (July 5, 2022)."Methane much more sensitive to global heating than previously thought – study".The Guardian.RetrievedJuly 5,2022.
- ^Global Methane Pledge."Homepage | Global Methane Pledge".www.globalmethanepledge.org.RetrievedAugust 2,2023.
- ^Forster, Piers; Smith, Chris; Rogelj, Joeri (November 2, 2021)."Guest post: The Global Methane Pledge needs to go further to help limit warming to 1.5C".Carbon Brief.RetrievedAugust 2,2023.
- ^IEA (2022)."Global Methane Tracker 2022".IEA.RetrievedAugust 2,2023.
- ^Bohrmann, Gerhard; Torres, Marta E. (2006), Schulz, Horst D.; Zabel, Matthias (eds.), "Gas Hydrates in Marine Sediments",Marine Geochemistry,Springer Berlin Heidelberg, pp. 481–512,doi:10.1007/3-540-32144-6_14,ISBN9783540321446
- ^Miller, G. Tyler (2007).Sustaining the Earth: An Integrated Approach.U.S.: Thomson Advantage Books, p. 160.ISBN0534496725
- ^Dean, J. F. (2018)."Methane feedbacks to the global climate system in a warmer world".Reviews of Geophysics.56(1): 207–250.Bibcode:2018RvGeo..56..207D.doi:10.1002/2017RG000559.hdl:1874/366386.
- ^abRuppel; Kessler (2017)."The interaction of climate change and methane hydrates".Reviews of Geophysics.55(1): 126–168.Bibcode:2017RvGeo..55..126R.doi:10.1002/2016RG000534.hdl:1912/8978.Archivedfrom the original on February 7, 2020.RetrievedSeptember 16,2019.
- ^"Methane Releases From Arctic Shelf May Be Much Larger and Faster Than Anticipated"(Press release). National Science Foundation (NSF). March 10, 2010.Archivedfrom the original on August 1, 2018.RetrievedApril 6,2018.
- ^Connor, Steve (December 13, 2011)."Vast methane 'plumes' seen in Arctic ocean as sea ice retreats".The Independent.Archivedfrom the original on December 25, 2011.RetrievedSeptember 4,2017.
- ^"Arctic sea ice reaches lowest extent for the year and the satellite record"(Press release). The National Snow and Ice Data Center (NSIDC). September 19, 2012.Archivedfrom the original on October 4, 2012.RetrievedOctober 7,2012.
- ^"Frontiers 2018/19: Emerging Issues of Environmental Concern".UN Environment.Archived fromthe originalon March 6, 2019.RetrievedMarch 6,2019.
- ^"Scientists shocked by Arctic permafrost thawing 70 years sooner than predicted".The Guardian.Reuters. June 18, 2019.ISSN0261-3077.Archivedfrom the original on October 6, 2019.RetrievedJuly 14,2019.
- ^Shindell, Drew; Kuylenstierna, Johan C. I.; Vignati, Elisabetta; van Dingenen, Rita; Amann, Markus; Klimont, Zbigniew; Anenberg, Susan C.; Muller, Nicholas; Janssens-Maenhout, Greet; Raes, Frank; Schwartz, Joel; Faluvegi, Greg; Pozzoli, Luca; Kupiainen, Kaarle; Höglund-Isaksson, Lena; Emberson, Lisa; Streets, David; Ramanathan, V.; Hicks, Kevin; Oanh, N. T. Kim; Milly, George; Williams, Martin; Demkine, Volodymyr; Fowler, David (January 13, 2012). "Simultaneously mitigating near-term climate change and improving human health and food security".Science.335(6065): 183–189.Bibcode:2012Sci...335..183S.doi:10.1126/science.1210026.ISSN1095-9203.PMID22246768.S2CID14113328.
- ^Dozolme, Philippe."Common Mining Accidents".About.com.Archivedfrom the original on November 11, 2012.RetrievedNovember 7,2012.
- ^Messina, Lawrence & Bluestein, Greg (April 8, 2010)."Fed official: Still too soon for W.Va. mine rescue".News.yahoo.com.Archivedfrom the original on April 8, 2010.RetrievedApril 8,2010.
- ^OSMAN, Karim; GENIAUT, Baptiste; HERCHIN, Nicolas; BLANCHETIERE, Vincent (2015)."A review of damages observed after catastrophic events experienced in the mid-stream gas industry compared to consequences modelling tools"(PDF).Symposium Series.160(25).RetrievedJuly 1,2022.
- ^Casal, Joaquim; Gómez-Mares, Mercedes; Muñoz, Miguel; Palacios, Adriana (2012)."Jet Fires: a" Minor "Fire Hazard?"(PDF).Chemical Engineering Transactions.26:13–20.doi:10.3303/CET1226003.RetrievedJuly 1,2022.
- ^"Porter Ranch gas leak permanently capped, officials say".Los Angeles Times.RetrievedFebruary 18,2016.
- ^Matt McGrath (February 26, 2016)."California methane leak 'largest in US history'".BBC.RetrievedFebruary 26,2016.
- ^Davila Fragoso, Alejandro (February 26, 2016)."The Massive Methane Blowout In Aliso Canyon Was The Largest in U.S. History".ThinkProgress.RetrievedFebruary 26,2016.
- ^Tim Walker (January 2, 2016)."California methane gas leak 'more damaging than Deepwater Horizon disaster'".The Independent.Archivedfrom the original on January 4, 2016.RetrievedJuly 6,2017.
- ^Carrington, Damian (May 9, 2023)."'Mind-boggling' methane emissions from Turkmenistan revealed ".The Guardian.RetrievedMay 9,2023.
- ^abZubrin, R. M.; Muscatello, A. C.; Berggren, M. (2013). "Integrated Mars in Situ Propellant Production System".Journal of Aerospace Engineering.26:43–56.doi:10.1061/(ASCE)AS.1943-5525.0000201.
- ^"Methane Blast".NASA. May 4, 2007.Archivedfrom the original on November 16, 2019.RetrievedJuly 7,2012.
- ^Chang, Kenneth (November 2, 2012)."Hope of Methane on Mars Fades".The New York Times.Archivedfrom the original on June 8, 2019.RetrievedNovember 3,2012.
- ^Atreya, Sushil K.; Mahaffy, Paul R.; Wong, Ah-San (2007). "Methane and related trace species on Mars: origin, loss, implications for life, and habitability".Planetary and Space Science.55(3): 358–369.Bibcode:2007P&SS...55..358A.doi:10.1016/j.pss.2006.02.005.hdl:2027.42/151840.
- ^Brown, Dwayne; Wendel, JoAnna; Steigerwald, Bill; Jones, Nancy; Good, Andrew (June 7, 2018)."Release 18-050 – NASA Finds Ancient Organic Material, Mysterious Methane on Mars".NASA.Archivedfrom the original on June 7, 2018.RetrievedJune 7,2018.
- ^NASA (June 7, 2018)."Ancient Organics Discovered on Mars – video (03:17)".NASA.Archivedfrom the original on June 7, 2018.RetrievedJune 7,2018.
- ^Wall, Mike (June 7, 2018)."Curiosity Rover Finds Ancient 'Building Blocks for Life' on Mars".Space.com.Archivedfrom the original on June 7, 2018.RetrievedJune 7,2018.
- ^Chang, Kenneth (June 7, 2018)."Life on Mars? Rover's Latest Discovery Puts It 'On the Table' – The identification of organic molecules in rocks on the red planet does not necessarily point to life there, past or present, but does indicate that some of the building blocks were present".The New York Times.Archivedfrom the original on June 8, 2018.RetrievedJune 8,2018.
- ^Voosen, Paul (June 7, 2018). "NASA rover hits organic pay dirt on Mars".Science.doi:10.1126/science.aau3992.S2CID115442477.
- ^ten Kate, Inge Loes (June 8, 2018). "Organic molecules on Mars".Science.360(6393): 1068–1069.Bibcode:2018Sci...360.1068T.doi:10.1126/science.aat2662.hdl:1874/366378.PMID29880670.S2CID46952468.
- ^Webster, Christopher R.; et al. (June 8, 2018)."Background levels of methane in Mars' atmosphere show strong seasonal variations".Science.360(6393): 1093–1096.Bibcode:2018Sci...360.1093W.doi:10.1126/science.aaq0131.PMID29880682.
- ^Eigenbrode, Jennifer L.;et al. (June 8, 2018)."Organic matter preserved in 3-billion-year-old mudstones at Gale crater, Mars".Science.360(6393): 1096–1101.Bibcode:2018Sci...360.1096E.doi:10.1126/science.aas9185.hdl:10044/1/60810.PMID29880683.
- ^Richardson, Derek (September 27, 2016)."Elon Musk Shows Off Interplanetary Transport System".Spaceflight Insider.Archivedfrom the original on October 1, 2016.RetrievedOctober 3,2016.
- ^Oze, C.; Sharma, M. (2005)."Have olivine, will gas: Serpentinization and the abiogenic production of methane on Mars".Geophysical Research Letters.32(10): L10203.Bibcode:2005GeoRL..3210203O.doi:10.1029/2005GL022691.S2CID28981740.
- ^"Cassini Explores a Methane Sea on Titan".Jet Propulsion Laboratory News.April 26, 2016.
- ^Committee on the Limits of Organic Life in Planetary Systems, Committee on the Origins and Evolution of Life, National Research Council;The Limits of Organic Life in Planetary Systems;The National Academies Press, 2007; page 74.
- ^McKay, C. P.; Smith, H. D. (2005)."Possibilities for methanogenic life in liquid methane on the surface of Titan".Icarus.178(1): 274–276.Bibcode:2005Icar..178..274M.doi:10.1016/j.icarus.2005.05.018.
- ^Volta, Alessandro (1777)Lettere del Signor Don Alessandro Volta... Sull' Aria Inflammable Nativa Delle PaludiArchivedNovember 6, 2018, at theWayback Machine[Letters of Signor Don Alessandro Volta... on the flammable native air of the marshes], Milan, Italy: Giuseppe Marelli.
- ^abMethane.BookRags.Archivedfrom the original on March 3, 2016.RetrievedJanuary 26,2012.
- ^Holland, John (1841).The history and description of fossil fuel, the collieries, and coal trade of Great Britain.London, Whittaker and Co. pp. 271–272.RetrievedMay 16,2021.
- ^Hofmann, A. W. (1866)."On the action of trichloride of phosphorus on the salts of the aromatic monoamines".Proceedings of the Royal Society of London.15:55–62.JSTOR112588.Archivedfrom the original on May 3, 2017.RetrievedJune 14,2016.;see footnote on pp. 57–58
- ^McBride, James Michael (1999)"Development of systematic names for the simple alkanes".Chemistry Department, Yale University (New Haven, Connecticut).ArchivedMarch 16, 2012, at theWayback Machine
- ^Harper, Douglas."methane".Online Etymology Dictionary.
- ^Jayasundara, Susantha (December 3, 2014)."Is there is any difference in expressing greenhouse gases as CH4Kg/ha and CH4-C Kg/ha?".ResearchGate.Archivedfrom the original on October 1, 2021.RetrievedAugust 26,2020.
- ^"User's Guide For Estimating Carbon Dioxide, Methane, And Nitrous Oxide Emissions From Agriculture Using The State Inventory Tool"(PDF).US EPA.November 26, 2019.Archived(PDF)from the original on October 1, 2021.RetrievedAugust 26,2020.
- ^"What does CH4-C mean? – Definition of CH4-C – CH4-C stands for Methane-carbon ratio".acronymsandslang.com.Archivedfrom the original on April 11, 2015.RetrievedAugust 26,2020.
- ^Office of Air and Radiation, US EPA (October 7, 1999)."U.S. Methane Emissions 1990–2020: Inventories, Projections, and Opportunities for Reductions (EPA 430-R-99-013)"(PDF).ourenergypolicy.org.Archived(PDF)from the original on October 26, 2020.RetrievedAugust 26,2020.
Cited sources
[edit]- Haynes, William M., ed. (2016).CRC Handbook of Chemistry and Physics(97th ed.).CRC Press.ISBN9781498754293.
External links
[edit]- MethaneatThe Periodic Table of Videos(University of Nottingham)
- International Chemical Safety Card 0291
- Gas (Methane) Hydrates – A New Frontier–United States Geological Survey(archived 6 February 2004)
- Lunsford, Jack H. (2000). "Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century".Catalysis Today.63(2–4): 165–174.doi:10.1016/S0920-5861(00)00456-9.
- CDC – Handbook for Methane Control in Mining(PDF)





