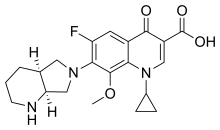
Moxifloxacin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Avelox, Vigamox, Moxiflox, others |
| Other names | Moxifloxacine; BAY 12-8039 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a600002 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth,intravenous,eye drops |
| Drug class | Antibiotic(fluoroquinolone) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokineticdata | |
| Bioavailability | 86%[2] |
| Protein binding | 47%[2] |
| Metabolism | Glucuronideandsulfateconjugation;CYP450system not involved[3] |
| Eliminationhalf-life | 12.1 hours[2] |
| Excretion | Urine,feces |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.129.459 |
| Chemical and physical data | |
| Formula | C21H24FN3O4 |
| Molar mass | 401.438g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Moxifloxacinis anantibiotic,used to treatbacterial infections,[4]includingpneumonia,conjunctivitis,endocarditis,tuberculosis,andsinusitis.[4][5]It can be given by mouth, byinjection into a vein,and as aneye drop.[5]
Common side effects includediarrhea,dizziness, and headache.[4]Severe side effects may include spontaneoustendon ruptures,nerve damage,and worsening ofmyasthenia gravis.[4]Safety of use inpregnancyandbreastfeedingis unclear.[6]Moxifloxacin is in thefluoroquinolonefamily of medications.[4]It usuallykills bacteriaby blocking their ability to duplicateDNA.[4]
Moxifloxacin was patented in 1988 and approved for use in the United States in 1999.[7][8]It is on theWorld Health Organization's List of Essential Medicines.[9]In 2021, it was the 286th most commonly prescribed medication in the United States, with more than 700,000 prescriptions.[10][11]
Medical uses
[edit]Moxifloxacin treats a number of infections, includingrespiratory-tract infections,bubonic plague,cellulitis,anthrax,intra-abdominal infections,endocarditis,meningitis,andtuberculosis.[12]
In the United States, moxifloxacin is licensed for the treatment of acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, community-acquired pneumonia, complicated and uncomplicated infections of the skin and of the skin structure, and complicated intra-abdominal infections.[13]In the European Union, it is licensed for acute bacterial exacerbations of chronic bronchitis, non-severe community-acquired pneumonia, and acute bacterial sinusitis. On the basis of its investigation into reports of rare but severe cases of liver toxicity and skin reactions, the European Medicines Agency recommended in 2008 that the use of the oral (but not the intravenous) form of moxifloxacin be restricted to infections in which other antibacterial agents cannot be used or have failed.[14]In the United States, the marketing approval does not contain these restrictions, though the label contains prominent warnings of skin reactions.
The initial approval by the Food and Drug Administration of the United States (December 1999)[15]encompassed these indications:
- Acute exacerbations of chronic bronchitis
- Acute bacterial sinusitis
- Community acquired pneumonia
Additional indications approved by the Food and Drug Administration:
- April 2001: Uncomplicated skin and skin-structure infections[16]
- May 2004: Community-acquired pneumonia caused by multidrug-resistantStreptococcus pneumoniae[17]
- June 2005: Complicated skin and skin-structure infections[18]
- November 2005: Complicated intra-abdominal infections[19]
TheEuropean Medicines Agencyhas advised that, for pneumonia, acute bacterial sinusitis, and acute exacerbations of COPD, it should only be used when other antibiotics are inappropriate.[20][21]
Oral and intravenous moxifloxacin have not been approved for children. Several drugs in this class, including moxifloxacin, are not licensed by the Food and Drug Administration for use in children, because of the risk of permanent injury to the musculoskeletal system.[22][23][24]Moxifloxacin eye drops are approved forconjunctivalinfections caused by susceptible bacteria.[25]
Recently, alarming reports of moxifloxacin resistance rates among anaerobes have been published. In Austria 36% ofBacteroideshave been reported to be resistant to moxifloxacin,[26]while in Italy resistance rates as high as 41% have been reported.[27]
Susceptible bacteria
[edit]A broad spectrum of bacteria is susceptible, including the following:
- Staphylococcus aureus
- Staphylococcus epidermidis
- Streptococcus pneumoniae
- Haemophilus influenzae
- Klebsiellaspp.
- Moraxella catarrhalis
- Enterobacterspp.
- Mycobacteriumspp.
- Bacillus anthracis
- Mycoplasma genitalium[28]
- Borrelia Burgdoferi(found to be effective in vitro)[29]
Adverse effects
[edit]Rare but serious adverse effects that may occur as a result of moxifloxacin therapy include irreversibleperipheral neuropathy,spontaneous tendon rupture andtendonitis,[30]hepatitis,psychiatric effects (hallucinations, depression),torsades de pointes,Stevens–Johnson syndromeandClostridium difficile-associated disease,[31]and photosensitivity/phototoxicity reactions.[32][33]
Several reports suggest the use of moxifloxacin may lead touveitis.[34]
Pregnancy and breastfeeding
[edit]Exposure of the developing fetus to quinolones, including levofloxacin, during the first-trimester is not associated with an increased risk of stillbirths, premature births, birth defects, or low birth weight.[35]There is limited data about the appearance of moxifloxacin in human breastmilk. Animal studies have found that moxifloxacin appears in significant concentration in breastmilk.[36]Decisions as to whether to continue therapy during pregnancy or while breast feeding should take the potential risk of harm to the fetus or child into account, as well as the importance of the drug to the well-being of the mother.[37]
Contraindications
[edit]Only two listed contraindications are found within the 2008 package insert:
- "Nonsteroidal anti-inflammatory drugs(NSAIDs): Although not observed with moxifloxacin in preclinical and clinical trials, the concomitant administration of a nonsteroidal anti-inflammatory drug with a fluoroquinolone may increase the risks ofCNSstimulation and convulsions. "[38]
- "Moxifloxacin is contraindicated in persons with a history of hypersensitivity to moxifloxacin, any member of the quinolone class of antimicrobial agents, or any of the product components."[38]
Though not stated as such within the package insert, ziprasidone is also considered to be contraindicated, as it may have the potential to prolong QT interval. Moxifloxacin should also be avoided in patients with uncorrected hypokalemia, or concurrent administration of other medications known to prolong the QT interval (antipsychotics and tricyclic antidepressants).[39]
Moxifloxacin should be used with caution in patients withdiabetes,as glucose regulation may be significantly altered.[39]
Moxifloxacin is also considered to be contraindicated within the pediatric population,pregnancy,nursing mothers, patients with a history of tendon disorder, patients with documented QT prolongation,[40]and patients withepilepsyor other seizure disorders. Coadministration of moxifloxacin with other drugs that also prolong the QT interval or induce bradycardia (e.g., beta-blockers, amiodarone) should be avoided. Careful consideration should be given in the use of moxifloxacin in patients with cardiovascular disease, including those with conduction abnormalities.[39]
Children and adolescents
[edit]The safety of moxifloxacin in human patients under age 18 has not been established. Animal studies suggest a risk of musculoskeletal harm in juveniles.[37]
Interactions
[edit]Moxifloxacin is not believed to be associated with clinically significant drug interactions due to inhibition or stimulation of hepatic metabolism. Thus, it should not, for the most part, require special clinical or laboratory monitoring to ensure its safety.[41]Moxifloxacin has a potential for a serious drug interaction with NSAIDs.[42]
The combination ofcorticosteroidsand moxifloxacin has increased potential to result in tendonitis and disability.[43]
Antacids containingaluminiumormagnesiumionsinhibit the absorption of moxifloxacin. Drugs that prolong theQT interval(e.g.,pimozide) may have an additive effect on QT prolongation and lead to increased risk of ventricular arrhythmias. Theinternational normalised ratiomay be increased or decreased in patients treated withwarfarin.[42]
Overdose
[edit]"In the event of acute overdose, the stomach should be emptied and adequate hydration maintained. ECG monitoring is recommended due to the possibility of QT interval prolongation. The patient should be carefully observed and given supportive treatment. The administration ofactivated charcoalas soon as possible after oral overdose may prevent excessive increase of systemic moxifloxacin exposure. About 3% and 9% of the dose of moxifloxacin, as well as about 2% and 4.5% of its glucuronide metabolite are removed by continuous ambulatory peritoneal dialysis and hemodialysis, respectively. "(Quoting from 29 December 2008 package insert for Avelox)[38]
Pharmacology
[edit]Mechanism of action
[edit]Moxifloxacin is abroad-spectrum antibioticthat is active against bothGram-positiveandGram-negativebacteria. It functions by inhibitingDNA gyrase,a type IItopoisomerase,and topoisomerase IV,[44]enzymes necessary to separate bacterial DNA, thereby inhibiting cell replication.
Pharmacokinetics
[edit]About 52% of an oral or intravenous dose of moxifloxacin is metabolized via glucuronide and sulfate conjugation. The cytochrome P450 system is not involved in moxifloxacin metabolism, and is not affected by moxifloxacin.[3]The sulfate conjugate (M1) accounts for around 38% of the dose, and is eliminated primarily in the feces. Approximately 14% of an oral or intravenous dose is converted to a glucuronide conjugate (M2), which is excreted exclusively in the urine. Peak plasma concentrations of M2 are about 40% those of the parent drug, while plasma concentrations of M1 are, in general, less than 10% those of moxifloxacin.[38]
In vitrostudies with cytochrome (CYP) P450 enzymes indicate that moxifloxacin does not inhibit 80 CYP3A4, CYP2D6, CYP2C9, CYP2C19, or CYP1A2, suggesting that moxifloxacin is unlikely to alter the pharmacokinetics of drugs metabolized by these enzymes.[38][3]
The pharmacokinetics of moxifloxacin in pediatric subjects have not been studied.[38]
Theelimination half-lifeof moxifloxacin is 11.5 to 15.6 hours (single-dose, oral).[45]About 45% of an oral or intravenous dose of moxifloxacin is excreted as unchanged drug (about 20% in urine and 25% in feces). A total of 96 ± 4% of an oral dose is excreted as either unchanged drug or known metabolites. The mean (± SD) apparent total body clearance and renal clearance are 12 ± 2 L/h and 2.6 ± 0.5 L/h, respectively.[45]The CSF penetration of moxifloxacin is 70% to 80% in patients withmeningitis.[46]
Chemistry
[edit]Moxifloxacin monohydrochloride is a slightly yellow to yellow crystalline substance.[38]It is synthesized in several steps, the first involving the preparation ofracemic2,8-diazabicyclo[4.3.0]nonane which is thenresolvedusingtartaric acid.A suitably derivatised quinolinecarboxylic acid is then introduced, in the presence ofDABCO,followed by acidification to form moxifloxacin hydrochloride.[47]
History
[edit]Moxifloxacin was first patented (United States patent) in 1991 by Bayer A.G., and again in 1997.[48]Avelox was subsequently approved by the U.S. Food and Drug Administration (FDA) for use in the United States in 1999 to treat specific bacterial infections.[7]Ranking 140th within the top 200 prescribed drugs in the United States for 2007,[49]Avelox generated sales of $697.3 million worldwide.[50]
Moxifloxacin is also manufactured byAlconas Vigamox.[51]
Patent
[edit]A United States patent application was made on 30 June 1989, for Avelox, Bayer A.G. being the assignee, which was subsequently approved on 5 February 1991. This patent was scheduled to expire on 30 June 2009. However, this patent was extended for an additional two and one half years on 16 September 2004, and as such was not expected to expire until 2012.[52] Moxifloxacin was subsequently (ten years later) approved by the FDA for use in the United States in 1999. At least four additional United States patents have been filed regarding moxifloxacin hydrochloride since the 1989 United States application,[48][53]as well as patents outside of the US.
Society and culture
[edit]Regulatory actions
[edit]Regulatory agencies have taken actions to address certain rare but serious adverse events associated with moxifloxacin therapy.[citation needed]
Based on its investigation into reports of rare but severe cases of liver toxicity and skin reactions, the European Medicines Agency recommended in 2008 that the use of the oral (but not the IV) form of moxifloxacin be restricted to infections in which other antibacterial agents cannot be used or have failed.[14]Similarly, the Canadian label includes a warning of the risk of liver injury.[54]
The U.S. label does not contain restrictions similar to the European label, but a carries a "black box" warning of the risk of tendon damage and/or rupture and warnings regarding the risk of irreversible peripheral neuropathy.[55]
Generic equivalents
[edit]In 2007, the U.S. District Court for the District of Delaware held that two Bayer patents on Avelox are valid and enforceable, and infringed by Dr. Reddy's ANDA for a generic version of Avelox.[56][57]The district court sided with Bayer, citing the Federal Circuit's prior decision inTakeda v. Alphapharm[58]as "affirming the district court's finding that defendant failed to prove a prima facie case of obviousness where theprior artdisclosed a broad selection of compounds, any one of which could have been selected as a lead compound for further investigation, and defendant did not prove that the prior art would have led to the selection of the particular compound singled out by defendant. "According to Bayer's press release[56]announcing the court's decision, it was noted that Teva had also challenged the validity of the same Bayer patents at issue in the Dr. Reddy's case. Within Bayer's first-quarter 2008 stockholder's newsletter[59]Bayer stated that they had reached an agreement with Teva Pharmaceuticals USA, Inc., the adverse party, to settle their patent litigation with regard to the two Bayer patents. Under the settlement terms agreed upon, Teva would obtain a license to sell its generic moxifloxacin tablet product in the U.S. shortly before the second of the two Bayer patents expires in March 2014. In Bangladesh, it is available with brand name of Optimox.[citation needed]
References
[edit]- ^"FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)".nctr-crs.fda.gov.FDA.Retrieved22 October2023.
- ^abcZhanel GG, Fontaine S, Adam H, Schurek K, Mayer M, Noreddin AM, et al. (2006). "A Review of New Fluoroquinolones: Focus on their Use in Respiratory Tract Infections".Treatments in Respiratory Medicine.5(6): 437–465.doi:10.2165/00151829-200605060-00009.PMID17154673.S2CID26955572.
- ^abcWorld Health Organization (2008).Guidelines for the Programmatic Management of Drug-resistant Tuberculosis.World Health Organization. pp. 189–.ISBN978-92-4-154758-1.
- ^abcdef"Moxifloxacin Hydrochloride".The American Society of Health-System Pharmacists.Retrieved29 August2017.
- ^abBritish national formulary: BNF 69(69 ed.). British Medical Association. 2015. pp. 408, 757.ISBN9780857111562.
- ^"Moxifloxacin Use During Pregnancy".Drugs.com.Retrieved10 December2017.
- ^ab"Details for NDA:021085".DrugPatentWatch.Retrieved17 July2009.
- ^Fischer J, Ganellin CR (2006).Analogue-based Drug Discovery.John Wiley & Sons. p. 501.ISBN9783527607495.
- ^World Health Organization(2023).The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023).Geneva: World Health Organization.hdl:10665/371090.WHO/MHP/HPS/EML/2023.02.
- ^"The Top 300 of 2021".ClinCalc.Archivedfrom the original on 15 January 2024.Retrieved14 January2024.
- ^"Moxifloxacin - Drug Usage Statistics".ClinCalc.Retrieved14 January2024.
- ^"Avelox".The American Society of Health-System Pharmacists.Retrieved3 April2011.
- ^"Documents"(PDF).accessdata.fda.gov.
- ^ab"Press release"(PDF).Europa (web portal).
- ^"Application letter"(PDF).accessdata.fda.gov.1999.Retrieved7 June2019.
- ^"Approval of supplements"(PDF).accessdata.fda.gov.2001.Retrieved7 June2019.
- ^"Application letter"(PDF).accessdata.fda.gov.2004.Retrieved7 June2019.
- ^"Approval of supplements"(PDF).accessdata.fda.gov.2005.Retrieved7 June2019.
- ^"Application letter"(PDF).accessdata.fda.gov.Retrieved7 June2019.
- ^"Moxifloxacin: increased risk of life-threatening liver reactions and other serious risks".gov.uk.Retrieved8 February2022.
- ^"European Medicines Agency recommends restricting the use of oral moxifloxacin-containing medicines"(PDF).European Medicines Agency. 24 July 2008. Archived fromthe original(PDF)on 8 July 2009.Retrieved20 July2009.
- ^"SYNOPSIS"(PDF).Retrieved29 January2009.
- ^Karande SC, Kshirsagar NA (February 1992). "Adverse drug reaction monitoring of ciprofloxacin in pediatric practice".Indian Pediatrics.29(2): 181–188.PMID1592498.
- ^Dolui SK, Das M, Hazra A (2007)."Ofloxacin-induced reversible arthropathy in a child".Journal of Postgraduate Medicine.53(2): 144–145.doi:10.4103/0022-3859.32220.PMID17495385.
- ^"Center for drug evaluation and research Application number 21-598"(PDF).Food and Drug Administration(FDA). 15 April 2005.Retrieved21 July2009.
- ^König E, Ziegler HP, Tribus J, Grisold AJ, Feierl G, Leitner E (April 2021)."Surveillance of Antimicrobial Susceptibility of Anaerobe Clinical Isolates in Southeast Austria:Bacteroides fragilisGroup Is on the Fast Track to Resistance ".Antibiotics.10(5): 479.doi:10.3390/antibiotics10050479.PMC8143075.PMID33919239.
- ^Principe L, Sanson G, Luzzati R, Aschbacher R, Pagani E, Luzzaro F, Di Bella S (August 2022). "Time to reconsider moxifloxacin anti-anaerobic activity?".Journal of Chemotherapy.35(4): 367–368.doi:10.1080/1120009X.2022.2106637.PMID35947127.S2CID251470489.
- ^Unemo M, Jensen JS (March 2017). "Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium".Nature Reviews. Urology.14(3): 139–152.doi:10.1038/nrurol.2016.268.PMID28072403.S2CID205521926.
- ^Pothineni VR, Wagh D, Babar MM, Inayathullah M, Solow-Cordero D, Kim KM, et al. (1 April 2016)."Identification of new drug candidates against Borrelia burgdorferi using high-throughput screening".Drug Design, Development and Therapy.10:1307–1322.doi:10.2147/DDDT.S101486.PMC4827596.PMID27103785.
- ^Albrecht R (28 July 2004)."NDA 21-085/S-024, NDA 21-277/S-019"(PDF).Center for Drug Evaluation and Research.Food and Drug Administration(FDA).Retrieved31 July2009.
- ^Albrecht R (31 May 2007)."NDA 21-085/S-036, NDA 21-277/S-030"(PDF).Center for Drug Evaluation and Research.Food and Drug Administration(FDA).Retrieved31 July2009.
- ^Albrecht R (15 February 2008)."NDA 21-085/S-038, NDA 21-277/S-031"(PDF).Division of Special Pathogen and Transplant Products.Food and Drug Administration(FDA).Retrieved31 July2009.
- ^DEPARTMENT OF HEALTH & HUMAN SERVICES (28 February 2008)."NDA 21-085/S-014, S-015, S-017"(PDF).Food and Drug Administration(FDA).Retrieved17 July2009.
- ^"Risk for Uveitis With Oral Moxifloxacin".JAMA Ophthalmology online. 2 October 2014.
- ^Ziv A, Masarwa R, Perlman A, Ziv D, Matok I (March 2018). "Pregnancy Outcomes Following Exposure to Quinolone Antibiotics - a Systematic-Review and Meta-Analysis".Pharmaceutical Research.35(5): 109.doi:10.1007/s11095-018-2383-8.PMID29582196.S2CID4724821.
- ^Balfour JA, Lamb HM (January 2000). "Moxifloxacin: a review of its clinical potential in the management of community-acquired respiratory tract infections".Drugs.59(1): 115–139.doi:10.2165/00003495-200059010-00010.PMID10718103.S2CID195694147.
- ^ab"merck.com"(PDF).
- ^abcdefgBayer (December 2008)."AVELOX (moxifloxacin hydrochloride) Tablets AVELOX I.V. (moxifloxacin hydrochloride in sodium chloride injection)"(PDF).Food and Drug Administration(FDA). p. 19.Retrieved2 November2010.
- ^abc"Moxifloxacin".University of Maryland Medical Center. 2009. Archived fromthe originalon 7 March 2009.Retrieved22 July2009.
- ^"Microsoft Word - Rote Hand Brief Avalox an BfArM.doc"(PDF).Retrieved7 June2019.
- ^Nightingale CH (March 2000). "Moxifloxacin, a new antibiotic designed to treat community-acquired respiratory tract infections: a review of microbiologic and pharmacokinetic-pharmacodynamic characteristics".Pharmacotherapy.20(3): 245–256.doi:10.1592/phco.20.4.245.34880.PMID10730681.S2CID11448163.
- ^abU.S. Food and Drug Administration(15 December 1999)."RE: NDA # 21-085 Avelox (moxifloxacin hydrochloride) Tablets MACMIS # 8577"(PDF).Letter to Martina Ziska. Archived fromthe original(PDF)on 9 March 2009.Retrieved11 April2009.
- ^Albrecht R (16 May 2002)."NDA 21-085/S-012"(PDF).Food and Drug Administration(FDA).Retrieved17 July2009.
- ^Drlica K, Zhao X (September 1997)."DNA gyrase, topoisomerase IV, and the 4-quinolones".Microbiology and Molecular Biology Reviews.61(3): 377–392.doi:10.1128/mmbr.61.3.377-392.1997.PMC232616.PMID9293187.
- ^ab"Drug card for Moxifloxacin (DB00218)".Canada: DrugBank. 19 February 2009.Retrieved3 August2009.
- ^Alffenaar JW, van Altena R, Bökkerink HJ, Luijckx GJ, van Soolingen D, Aarnoutse RE, van der Werf TS (October 2009)."Pharmacokinetics of moxifloxacin in cerebrospinal fluid and plasma in patients with tuberculous meningitis".Clinical Infectious Diseases.49(7): 1080–1082.doi:10.1086/605576.hdl:2066/79494.PMID19712035.
- ^Peterson U (2006)."Quinolone Antibiotics: The Development of Moxifloxacin".In Fischer J, Ganellin CR (eds.).Analogue-based Drug Discovery.John Wiley & Sons.pp. 338–342.ISBN9783527607495.
- ^ab"Inventors/Applicants".patentlens.net. 3 October 2006. Archived fromthe originalon 21 February 2013.Retrieved17 July2009.
- ^Lamb E (1 May 2008)."Top 200 Prescription Drugs of 2007".Pharmacy Times.Archived fromthe originalon 7 February 2009.Retrieved21 July2009.
- ^"EU agency recommends restricting moxifloxacin use".Reuters.24 July 2008.Retrieved21 July2009.
- ^"Alcon's Newest Antibiotic, Vigamox Ophthalmic Solution, Earns FDA Approval".Infection Control Today.Alcon.22 June 2003. Archived fromthe originalon 16 August 2016.Retrieved25 June2016.
- ^"Patent form"(PDF).uspto.gov.1 February 1991.Retrieved7 June2019.
- ^US 4990517 A (Feb 1991) Petersenet al.See[1]US 5051509 A (Sep 1991) Naganoet al.See[2]US 5059597 A (Oct 1991) Petersenet al.See[3]US 5395944 A (Mar 1995) Petersenet al.See[4]US 5416096 A (May 1995) Petersenet al.See[5]
- ^"Updated Labelling for Antibiotic Avelox (Moxifloxacin) Regarding Rare Risk of Severe Liver Injury".Health Canada.22 March 2010.
Information Update: 2010-42
- ^Albrecht R (3 October 2008)."NDA 21-085/S-040, NDA 21-277/S-034"(PDF).Center for Drug Evaluation and Research.Food and Drug Administration.Retrieved31 July2009.
- ^abBayer AG(6 November 2007)."Ruling in Bayer's favor over Avelox patents".Bayer. Archived fromthe originalon 11 December 2008.Retrieved29 August2009.
- ^"Opinion form"(PDF).orangebookblog.com.5 October 2007.Retrieved7 June2019.
- ^"United States Court of Appeals for the Federal Circuit"(PDF).uscourts.gov. 28 June 2007. Archived fromthe original(PDF)on 26 August 2009.Retrieved29 August2009.
- ^Bayer AG(24 April 2008)."Risk Report".Bayer. Archived fromthe originalon 9 March 2009.Retrieved29 August2009.
