From Wikipedia, the free encyclopedia
First generation antihistamine
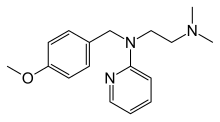
Mepyramine ,also known aspyrilamine ,is afirst generation antihistamine ,targeting theH1 receptor as aninverse agonist .[ 1] drowsiness .[ 2] maleate salt,pyrilamine maleate .
The medication has negligibleanticholinergic activity, with 130,000-foldselectivity for thehistamine H1 receptor over themuscarinic acetylcholine receptors (for comparison,diphenhydramine had 20-fold selectivity for the H1 receptor).[ 3]
It was patented in 1943 and came into medical use in 1949.[ 4] [ 5] Sominex ,Nytol, and many others.[ 5] FDA included it in the list of chemicals and compounds barred from use inover-the-counter nighttime sleep aid products in 1989.[ 6]
It is used in over-the-counter combination products to treat the common cold and menstrual symptoms such asMidol Complete .[ 7] [ 8] [ 1]
Psychedelics (5-HT2A
Benzofurans Lyserg‐ Phenethyl‐
2C-x
3C-x 4C-x DOx HOT-x MDxx Mescaline (subst.) TMAs
TMA
TMA-2
TMA-3
TMA-4
TMA-5
TMA-6 Others
Piperazines Tryptamines
alpha -alkyltryptaminesx -DALT x -DET x -DiPT x -DMT
4,5-DHP-DMT 2,N,N-TMT 4-AcO-DMT 4-HO-5-MeO-DMT 4,N,N-TMT 4-Propionyloxy-DMT 5,6-diBr-DMT 5-AcO-DMT 5-Bromo-DMT 5-MeO-2,N ,N -TMT 5-MeO-4,N ,N -TMT 5-MeO-α,N,N-TMT 5-MeO-DMT 5-N ,N -TMT 7,N,N-TMT α,N,N-TMT (Bufotenin) 5-HO-DMT DMT Norbaeocystin (Psilocin) 4-HO-DMT (Psilocybin) 4-PO-DMT x -DPT Ibogaine-related x -MET x -MiPT Others
Others
Dissociatives (NMDAR antagonists )
Deliriants (mAChR antagonists ) Others
H1
Agonists Antagonists
Others: Atypical antipsychotics (e.g.,aripiprazole ,asenapine ,brexpiprazole ,brilaroxazine ,clozapine ,iloperidone ,olanzapine ,paliperidone ,quetiapine ,risperidone ,ziprasidone ,zotepine )Phenylpiperazine antidepressants (e.g.,hydroxynefazodone ,nefazodone ,trazodone ,triazoledione )Tetracyclic antidepressants (e.g.,amoxapine ,loxapine ,maprotiline ,mianserin ,mirtazapine ,oxaprotiline )Tricyclic antidepressants (e.g.,amitriptyline ,butriptyline ,clomipramine ,desipramine ,dosulepin (dothiepin) ,doxepin ,imipramine ,iprindole ,lofepramine ,nortriptyline ,protriptyline ,trimipramine )Typical antipsychotics (e.g.,chlorpromazine ,flupenthixol ,fluphenazine ,loxapine ,perphenazine ,prochlorperazine ,thioridazine ,thiothixene )
H2
H3
H4
DAT Tooltip Dopamine transporter (DRIs Tooltip Dopamine reuptake inhibitors )
NET Tooltip Norepinephrine transporter (NRIs Tooltip Norepinephrine reuptake inhibitors )
Others: Antihistamines (e.g.,brompheniramine ,chlorphenamine ,pheniramine ,tripelennamine )Antipsychotics (e.g.,loxapine ,ziprasidone )Arylcyclohexylamines (e.g.,ketamine ,phencyclidine )Dopexamine Ephenidine Ginkgo biloba Indeloxazine Nefazodone Opioids (e.g.,desmetramadol ,methadone ,pethidine (meperidine) ,tapentadol ,tramadol ,levorphanol )
SERT Tooltip Serotonin transporter (SRIs Tooltip Serotonin reuptake inhibitors )
Others: A-80426 Amoxapine Antihistamines (e.g.,brompheniramine ,chlorphenamine ,dimenhydrinate ,diphenhydramine ,mepyramine (pyrilamine) ,pheniramine ,tripelennamine )Antipsychotics (e.g.,loxapine ,ziprasidone )Arylcyclohexylamines (e.g.,3-MeO-PCP ,esketamine ,ketamine ,methoxetamine ,phencyclidine )Cyclobenzaprine Delucemine Dextromethorphan Dextrorphan Efavirenz Hypidone Medifoxamine Mesembrine Mifepristone MIN-117 (WF-516) N-Me-5-HT Opioids (e.g.,dextropropoxyphene ,methadone ,pethidine (meperidine) ,levorphanol ,tapentadol ,tramadol )Roxindole
VMATs Tooltip Vesicular monoamine transporters Others