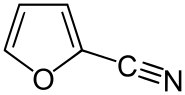
2-Furonitrile
| |
| Names | |
|---|---|
| Preferred IUPAC name
Furan-2-carbonitrile | |
| Other names
2-Cyanofuran; 2-Furancarbonitrile; 2-Furyl cyanide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.009.581 |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C5H3NO | |
| Molar mass | 93.085g·mol−1 |
| Appearance | colorless (yellow if impure) |
| Density | 1.0650 @20 °C[1] |
| Boiling point | 147[2]°C (297 °F; 420 K) |
| Hazards | |
| Flash point | 35 °C; 95 °F; 308 K |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
2-Furonitrileis a colorless derivative offuranpossessing anitrilegroup.
Synthesis
[edit]Industrial synthesis is based on the vapor phaseammoxidationoffurfuralwithammoniaover bismuth molybdate catalyst at 440-480 °C.[3]
Numerous laboratory methods also exist; for the instance oxidative dehydration of furfural withammoniasalts usinghypervalent iodinereagents[4]orn-bromosuccinimide.[5]From furfuralaldoxime(with thionyl chloride-benzotriazole,[6]triphenylphosphine-iodine reagents,[7]or heating inDMSO[8]) and furoic acid amide (flash vacuum pyrolysis).[9]
Applications
[edit]2-Furonitrile currently has no major applications but it is used as an intermediate in pharmaceutical and fine chemical synthesis. It has been suggested as a potential sweetening agent, as it has about 30 times the sweetening power ofsucrose.[10]
References
[edit]- ^P. A. Pavlov; Kul'nevich, V. G. (1986). "Synthesis of 5-substituted furannitriles and their reaction with hydrazine".Khimiya Geterotsiklicheskikh Soedinenii.2:181–186.
- ^Patrice Capdevielle; Lavigne, Andre; Maumy, Michel (1989). "Simple and efficient copper-catalyzed one-pot conversion of aldehydes into nitriles".Synthesis.6(6): 451–452.doi:10.1055/s-1989-27285.S2CID97316774.
- ^Thomas J. Jennings, "Process for preparing furonitrile", US Patent 3,260,731 (1966)
- ^Chenjie Zhu; Sun, Chengguo; Wei, Yunyang (2010). "Direct oxidative conversion of alcohols, aldehydes and amines into nitriles using hypervalent iodine(III) reagent".Synthesis.2010(24): 4235–4241.doi:10.1055/s-0030-1258281.
- ^Bandgar, B. P.; Makone, S. S. (2006). "Organic Reactions in Water: Transformation of Aldehydes to Nitriles using NBS under Mild Conditions".Synthetic Communications.36(10): 1347–1352.doi:10.1080/00397910500522009.ISSN0039-7911.S2CID98593006.
- ^Sachin S. Chaudhari; Akamanchi, Krishnacharya G. (1999). "Thionyl chloride-benzotriazole: an efficient system for transformation of aldoximes to nitriles".Synthetic Communications.29(10): 1741–1745.doi:10.1080/00397919908086161.
- ^A. Narsaiah; Sreenu, D.; Nagaiah, K. (2006). "Triphenylphosphine-iodine. An efficient reagent system for the synthesis of nitriles from aldoximes".Synthetic Communications.36(2): 137–140.doi:10.1080/00397910500333225.
- ^Aspinall, Helen C.; Beckingham, Oliver; Farrar, Michael D.; Greeves, Nicholas; Thomas, Christopher D. (2011). "A general and convenient route to oxazolyl ligands".Tetrahedron Letters.52(40): 5120–5123.doi:10.1016/j.tetlet.2011.07.070.ISSN0040-4039.
- ^Jacqueline A. Campbell; McDougald, Graham; McNab, Hamish (2007). "Laboratory-scale synthesis of nitriles by catalyzed dehydration of amides and oximes under flash vacuum pyrolysis (FVP) conditions".Synthesis.2007(20): 3179–3184.doi:10.1055/s-2007-990782.
- ^Thomas J. Jennings, "Process for preparing furonitrile", US Patent 3,260,731 (1966)
