Prokaryotic large ribosomal subunit
This articleneeds additional citations forverification.(October 2011) |
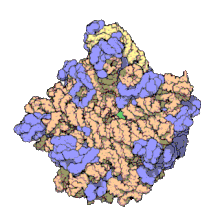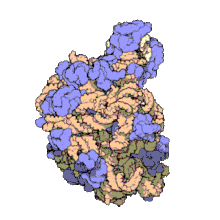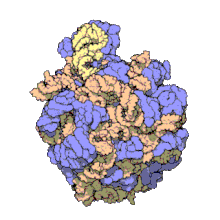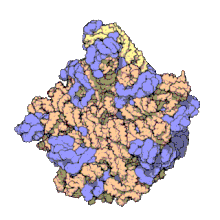
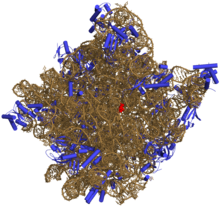
50Sis the larger subunit of the70Sribosomeofprokaryotes,i.e.bacteriaandarchaea.It is the site of inhibition forantibioticssuch asmacrolides,chloramphenicol,clindamycin,and thepleuromutilins.It includes the5S ribosomal RNAand23S ribosomal RNA.
Despite having the same sedimentation rate, bacterial and archaeal ribosomes can be quite different.
Structure[edit]
50S, roughly equivalent to the60Sribosomal subunit ineukaryoticcells, is the larger subunit of the70S ribosomeof prokaryotes. The 50S subunit is primarily composed of proteins but also contains single-strandedRNAknown asribosomal RNA(rRNA). rRNA forms secondary and tertiary structures to maintain the structure and carry out the catalytic functions of the ribosome.
X-ray crystallographyhas yieldedelectron density mapsallowing the structure of the 50S inHaloarcula marismortui(archaeon) to be determined to 2.4Åresolution[1]and of the 50S in theDeinococcus radiodurans(bacterium) to 3.3Å.[2]The large ribosomal subunit (50S) is approximately twice as massive as the small ribosomal subunit (30S). The model of Hm 50S, determined in 2000 byNenad Banand colleagues in the laboratory ofThomas Steitzand the laboratory ofPeter Moore,includes 2711 of the 2923nucleotidesof 23SrRNA,all 122 nucleotides of its 5S rRNA, and structure of 27 of its 31proteins.[1]
Ribosomal RNA[edit]
Thesecondary structureof 23S is divided into six large domains, within which domain V is most important in itspeptidyl transferase [3]activity. Each domain contains normal secondary structure (e.g., base triple, tetraloop, cross-strand purine stack) and is also highly symmetric in tertiary structure; proteins intervene between their helices. Attertiary structurelevel, the large subunit rRNA is a single gigantic domain while the small subunit contains three structural domains. This difference reflects the lesser flexibility of the large subunit required by its function. While its core is conserved, it accommodates expansion segments on its periphery.[4][5]
Difference between bacteria and archaeal versions[edit]
AcryoEMstructure of the 50S subunit from the archaeonMethanothermobacter thermautotrophicushas been determined. It shares the 50S size/sedimentation rate and the two rRNA count, but its 23S expansion segments have more in common with eukaryotes.[6]
A cryoEM reconstruction of the native 50S subunit of the extremely halophilic ArchaeanHalococcus morrhuae(classified underEuryarchaeota;Stenosarchaeagroup) is available. The 50S subunit contains a 108‐nucleotide insertion in its 5S rRNA,[7]which at subnanometer resolution, is observed to emerge from a four‐way junction without affecting the parental canonical 5S rRNA structure.[4]
Due to the differences, archaeal 50S are less sensitive to some antibiotics that target bacterial 50S.[8][9]
Function[edit]
50S includes the activity that catalyzespeptide bondformation (peptidyl transfer reaction), prevents premature polypeptide hydrolysis, provides a binding site for the G-protein factors (assistsinitiation,elongation,and termination), and helpsprotein foldingafter synthesis.
Promotes the peptidyl transfer reaction and prevents peptidyl hydrolysis[edit]
An induced-fit mechanism has been revealed for how 50S catalyzes the peptidyl transfer reaction and prevents peptidyl hydrolysis. Theamino groupof an aminoacyl-tRNA(binds to A site) attacks the carbon of acarbonylgroup of a peptidyl-tRNA (binds to P site) and finally yields a peptide extended by oneamino acidesterified to the A site tRNA bound to the ribosomal A site and a deacylated tRNA in the P site.
When the A site is unoccupied, nucleotide U2620 (E. coli U2585), A2486 (2451) and C2106 (2063) sandwich the carbonyl group in the middle, forcing it into an orientation facing the A site. This orientation prevents anynucleophilic attackfrom the A site because the optimal attacking angle is 105 degrees from the plane of theestergroup. When a tRNA with a complete[?] CCA sequence at its acceptor stem is bound to the A site, C74 of the tRNA stacking with U2590 (2555) induces a conformational change in the ribosome, resulting in movement of U2541 (2506), U2620 (2585) through G2618 (2583). The displacement of bases allows the ester group to adopt a new conformation accessible to nucleophilic attack from the A site.
The N3 (nitrogen) of A2486 (2451) is closest to the peptide bond being synthesized and may function as a general base to facilitate the nucleophilic attack by the amino group of the aminoacyl-tRNA (in the A site). The pKa of A2486 (2451) is about 5 units higher in order tohydrogen bondwith the amino group thus increasing its nucleophilicity. The elevation of pKa is achieved through a charge relay mechanism. A2486 (2451) interacts with G2482 (G2447), which hydrogen bonds with the buriedphosphateof A2486 (2450). This buried phosphate can stabilize the normally rare imino tautomers of both bases, resulting in an increase in the negative charge density on N3.
Helps protein formation[edit]
After initiation, elongation, and termination, there is a fourth step of the disassembly of the post-termination complex of ribosome,mRNA,and tRNA, which is a prerequisite for the next round of protein synthesis. The large ribosomal subunit has a role in protein folding bothin vitroandin vivo.The large ribosomal subunit provides ahydrophobicsurface for the hydrophobic collapse step of protein folding. The newly synthesized protein needs full access to the large subunit to fold; this process may take a period of time (5 minutes forbeta-galactosidase[citation needed]).
See also[edit]
References[edit]
- ^abcNissen, P.; Hansen, J.; Ban, N.; Moore, P.; Steitz, T. (2000). "The complete atomic structure of the large ribosomal subunit at 2.4 A resolution".Science.289(5481): 905–920.Bibcode:2000Sci...289..905B.CiteSeerX10.1.1.58.2271.doi:10.1126/science.289.5481.905.PMID10937989.
- ^Schluenzen, F.; Tocilj, A.; Zarivach, R.; Harms, J.; Gluehmann, M.; Janell, D.; Bashan, A.; Bartels, H.; Agmon, I.; Franceschi, F.; Yonath, A. (2000)."Structure of functionally activated small ribosomal subunit at 3.3 Å resolution".Cell.102(5): 615–623.doi:10.1016/S0092-8674(00)00084-2.PMID11007480.
- ^Tirumalai MR, Rivas M, Tran Q, Fox GE (November 10, 2021)."The Peptidyl Transferase Center: a Window to the Past".Microbiol Mol Biol Rev.85(4): e0010421.doi:10.1128/MMBR.00104-21.PMC8579967.PMID34756086.
- ^abTirumalai, MR; Kaelber, JT; Park, DR; Tran, Q; Fox, GE (31 August 2020)."Cryo-electron microscopy visualization of a large insertion in the 5S ribosomal RNA of the extremely halophilic archaeonHalococcus morrhuae".FEBS Open Bio.10(10): 1938–1946.doi:10.1002/2211-5463.12962.PMC7530397.PMID32865340.
- ^Penev PI, Fakhretaha-Aval S, Patel VJ, Cannone JJ, Gutell RR, Petrov AS, Williams LD, Glass JB (August 2020)."Supersized ribosomal RNA expansion segments in Asgard archaea".Genome Biology and Evolution.12(10): 1694–1710.doi:10.1093/gbe/evaa170.PMC7594248.PMID32785681.
- ^Greber, Basil J.; Boehringer, Daniel; Godinic-Mikulcic, Vlatka; Crnkovic, Ana; Ibba, Michael; Weygand-Durasevic, Ivana; Ban, Nenad (May 2012)."Cryo-EM Structure of the Archaeal 50S Ribosomal Subunit in Complex with Initiation Factor 6 and Implications for Ribosome Evolution".Journal of Molecular Biology.418(3–4): 145–160.doi:10.1016/j.jmb.2012.01.018.PMC3879142.PMID22306461.
- ^Luehrsen, KR.; Nicholson, DE; Eubanks, DC; Fox, GE (May 1981). "An archaebacterial 5S rRNA contains a long insertion sequence".Nature.293(5835): 755–756.Bibcode:1981Natur.293..755L.doi:10.1038/293755a0.PMID6169998.S2CID4341755.
- ^Khelaifia, S.; Drancourt, M. (September 2012)."Susceptibility of archaea to antimicrobial agents: applications to clinical microbiology".Clinical Microbiology and Infection.18(9): 841–848.doi:10.1111/j.1469-0691.2012.03913.x.PMID22748132.
- ^Thombre, Rebecca S.; Shinde, Vinaya; Thaiparambil, Elvina; Zende, Samruddhi; Mehta, Sourabh (13 September 2016)."Antimicrobial Activity and Mechanism of Inhibition of Silver Nanoparticles against Extreme Halophilic Archaea".Frontiers in Microbiology.7:1424.doi:10.3389/fmicb.2016.01424.PMC5020055.PMID27679615.
The haloarchaea used in the current study were resistant to nalidixic acid, streptomycin, gentamicin, tetracycline, erythromycin, chloramphenicol, cephalothin, and clindamycin.
- Nissen, P.; Hansen, J.; Ban, N.; Moore, P.; Steitz, T. (2000). "The structural basis of ribosome activity in peptide bond synthesis".Science.289(5481): 920–929.Bibcode:2000Sci...289..920N.doi:10.1126/science.289.5481.920.PMID10937990.
- Schmeing, T.; Huang, K.; Strobel, S.; Steitz, T. (2005). "An induced-fit mechanism to promote peptide bond formation and exclude hydrolysis of peptidyl-tRNA".Nature.438(7067): 520–524.Bibcode:2005Natur.438..520M.doi:10.1038/nature04152.PMID16306996.S2CID4333559.
- Basu, A.; Ghosh, J.; Bhattacharya, A.; Pal, S.; Chowdhury, S.; DasGupta, C. (2003). "Splitting of ribosome into its subunits by unfolded polypeptide chains".Current Science.84:1123–1125.
External links[edit]
- http://pathmicro.med.sc.edu/mayer/antibiot.htm
- https://web.archive.org/web/20110227235620/http://www.molgen.mpg.de/~ag_ribo/ag_franceschi/franceschi-projects-50S-antibiotics.html
- https://web.archive.org/web/20080206051722/http://www.riboworld.com/antib/50santib-eng.shtml
- 23S+Ribosomal+RNAat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
- 5S+Ribosomal+RNAat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
