Protein kinase B
| AKT1 | |||||||
|---|---|---|---|---|---|---|---|
 Ribbon Representation of crystal structure of Akt-1-inhibitor complexes.[1] | |||||||
| Identifiers | |||||||
| Symbol | AKT1 | ||||||
| NCBI gene | 207 | ||||||
| HGNC | 391 | ||||||
| OMIM | 164730 | ||||||
| RefSeq | NM_005163 | ||||||
| UniProt | P31749 | ||||||
| Other data | |||||||
| Locus | Chr. 14q32.32-32.33 | ||||||
| |||||||
| AKT2 | |||||||
|---|---|---|---|---|---|---|---|
 Crystal structure of Akt-2-inhibitor complexes.[2] | |||||||
| Identifiers | |||||||
| Symbol | AKT2 | ||||||
| NCBI gene | 208 | ||||||
| HGNC | 392 | ||||||
| OMIM | 164731 | ||||||
| RefSeq | NM_001626 | ||||||
| UniProt | P31751 | ||||||
| Other data | |||||||
| Locus | Chr. 19q13.1-13.2 | ||||||
| |||||||
| AKT3 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | AKT3 | ||||||
| NCBI gene | 10000 | ||||||
| HGNC | 393 | ||||||
| OMIM | 611223 | ||||||
| RefSeq | NM_181690 | ||||||
| UniProt | Q9Y243 | ||||||
| Other data | |||||||
| Locus | Chr. 1q43-44 | ||||||
| |||||||
Protein kinase B(PKB), also known asAkt,is the collective name of a set of threeserine/threonine-specific protein kinasesthat play key roles in multiple cellular processes such asglucose metabolism,apoptosis,cell proliferation,transcription,andcell migration.
Family members - Isoforms
[edit]There are three different genes that encodeisoformsof protein kinase B. These three genes are referred to asAKT1,AKT2,andAKT3and encode the RAC alpha, beta, and gamma serine/threonine protein kinases respectively. The termsPKBandAktmay refer to the products of all three genes collectively, but sometimes are used to refer to PKB alpha and Akt1 alone.[citation needed]
Akt1is involved in cellular survival pathways, by inhibitingapoptoticprocesses. Akt1 is also able to induceprotein synthesispathways, and is therefore a key signaling protein in the cellular pathways that lead to skeletal muscle hypertrophy and general tissue growth. A mouse model with complete deletion of the Akt1 gene manifests growth retardation and increased spontaneous apoptosis in tissues such as testes and thymus.[3]Since it can block apoptosis and thereby promote cell survival, Akt1 has been implicated as a major factor in many types of cancer.[4]Akt1 is also a positive regulator of cell migration.[5]Akt1 was originally identified as theoncogenein the transformingretrovirus,AKT8.[6]
Akt2is an important signaling molecule in theinsulin signaling pathway.It is required to induce glucose transport. In a mouse which is null for Akt1 but normal for Akt2, glucose homeostasis is unperturbed, but the animals are smaller, consistent with a role for Akt1 in growth. In contrast, mice which do not have Akt2, but have normal Akt1, have mild growth deficiency and display adiabeticphenotype (insulin resistance), again consistent with the idea that Akt2 is more specific for theinsulin receptorsignaling pathway.[7]Akt2 promotes cell migration as well.[5] The role ofAkt3is less clear, though it appears to be predominantly expressed in the brain. It has been reported that mice lacking Akt3 have small brains.[8]
Akt isoforms are overexpressed in a variety of human tumors, and, at the genomic level, are amplified in gastric adenocarcinomas (Akt1), ovarian (Akt2), pancreatic (Akt2) and breast (Akt2) cancers.[9][10]
Name
[edit]The name Akt does not refer to its function. The "Ak" in Akt refers to the AKR mouse strain that develops spontaneous thymic lymphomas. The "t" stands for 'thymoma'; the letter was added when a transforming retrovirus was isolated from the Ak mouse strain, which was termed "Akt-8". The authors state, "Stock A Strain k AKR mouse originally inbred in the laboratory of Dr. C. P. Rhoads by K. B. Rhoads at the Rockefeller Institute." When the oncogene encoded in this virus was discovered, it was termed v-Akt. Thus, the more recently identified human analogs were named accordingly.[11]
Regulation
[edit]Akt1 is involved in thePI3K/AKT/mTOR pathwayand other signaling pathways.[5]
Binding phospholipids
[edit]The Akt proteins possess aprotein domainknown as a PH domain, orpleckstrin homology domain,named afterpleckstrin,the protein in which it was first discovered. This domain binds tophosphoinositideswith high affinity. In the case of the PH domain of the Akt proteins, it binds either PIP3(phosphatidylinositol (3,4,5)-trisphosphate,PtdIns(3,4,5)P3) or PIP2(phosphatidylinositol (3,4)-bisphosphate,PtdIns(3,4)P2).[12]This is useful for control of cellular signaling because the di-phosphorylated phosphoinositidePIP2is only phosphorylated by the family of enzymes, PI 3-kinases (phosphoinositide 3-kinaseor PI3-K), and only upon receipt of chemical messengers which tell the cell to begin the growth process. For example, PI 3-kinases may be activated by aG protein coupled receptororreceptor tyrosine kinasesuch as theinsulin receptor.Once activated, PI 3-kinase phosphorylates PIP2to form PIP3.
Phosphorylation
[edit]Once correctly positioned at the membrane via binding ofPIP3,Akt can then be phosphorylated by its activating kinases, phosphoinositide-dependent kinase-1 (PDPK1at threonine 308 in Akt1 and threonine 309 in Akt2) and the mammalian target of rapamycin complex 2 (mTORC2at serine 473 (Akt1) and 474 (Akt2)) which is found at high levels in the fed state,[13][14]first by mTORC2. mTORC2 therefore functionally acts as the long-sought PDK2 molecule, although other molecules, includingintegrin-linked kinase(ILK) and mitogen-activated protein kinase-activated protein kinase-2 (MAPKAPK2) can also serve as PDK2. Phosphorylation by mTORC2 stimulates the subsequent phosphorylation of Akt isoforms by PDPK1.
Activated Akt isoforms can then go on to activate or deactivate their myriad substrates (e.g.mTOR) via their kinase activity.
Besides being a downstream effector of PI 3-kinases, Akt isoforms can also be activated in a PI 3-kinase-independent manner.[15]ACK1orTNK2,a non-receptor tyrosine kinase, phosphorylates Akt at its tyrosine 176 residue, leading to its activation in PI 3-kinase-independent manner.[15]Studies have suggested thatcAMP-elevating agents could also activate Akt throughprotein kinase A(PKA) in the presence of insulin.[16]
O-GlcNAcylation
[edit]Akt can beO-GlcNAcylatedbyOGT.O-GlcNAcylation of Akt is associated with a decrease in T308 phosphorylation.[17]
Ubiquitination
[edit]Akt1 is normallyphosphorylatedat position T450 in the turn motif when Akt1 is translated. If Akt1 is not phosphorylated at this position, Akt1 does not fold in the right way. The T450-non-phosphorylated misfolded Akt1 isubiquitinatedand degraded by theproteasome.Akt1 is also phosphorylated at T308 and S473 duringIGF-1response, and the resulting polyphosphorylated Akt is ubiquitinated partly byE3 ligaseNEDD4.Most of the ubiquitinated-phosphorylated-Akt1 is degraded by the proteasome, while a small amount of phosphorylated-Akt1 translocates to the nucleus in a ubiquitination-dependent way to phosphorylate its substrate. A cancer-derived mutant Akt1 (E17K) is more readily ubiquitinated and phosphorylated than the wild type Akt1. The ubiquitinated-phosphorylated-Akt1 (E17K) translocates more efficiently to the nucleus than the wild type Akt1. This mechanism may contribute to E17K-Akt1-induced cancer in humans.[18]
Lipid phosphatases and PIP3
[edit]PI3K-dependent Akt1 activation can be regulated through thetumor suppressorPTEN,which works essentially as the opposite ofPI3Kmentioned above.[19]PTEN acts as aphosphataseto dephosphorylatePIP3back toPIP2.This removes the membrane-localization factor from theAktsignaling pathway. Without this localization, the rate ofAkt1activation decreases significantly, as do all of the downstream pathways that depend onAkt1for activation.
PIP3can also be de-phosphorylated at the "5" position by the SHIP family of inositol phosphatases,SHIP1andSHIP2.These poly-phosphate inositol phosphatases dephosphorylatePIP3to formPIP2.
Protein phosphatases
[edit]The phosphatases in thePHLPPfamily,PHLPP1andPHLPP2have been shown to directly de-phosphorylate, and therefore inactivate, distinct Akt isoforms. PHLPP2 dephosphorylates Akt1 and Akt3, whereas PHLPP1 is specific for Akt2 and Akt3.[citation needed]
Function
[edit]The Akt kinases regulate cellular survival[20]andmetabolismby binding and regulating many downstream effectors, e.g.Nuclear Factor-κB,Bcl-2 family proteins, master lysosomal regulatorTFEBand murine double minute 2 (MDM2).
Cell survival
[edit]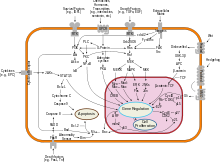
Akt kinases can promote growth factor-mediated cell survival both directly and indirectly.BADis a pro-apoptotic protein of theBcl-2family. Akt1 can phosphorylate BAD on Ser136,[21]which makes BAD dissociate from the Bcl-2/Bcl-X complex and lose the pro-apoptotic function.[22]Akt1 can also activateNF-κBvia regulatingIκB kinase(IKK), thus result in transcription of pro-survival genes.[23]
Cell cycle
[edit]The Akt isoforms are known to play a role in thecell cycle.Under various circumstances, activation of Akt1 was shown to overcome cell cycle arrest in G1[24]and G2[25]phases. Moreover, activated Akt1 may enable proliferation and survival of cells that have sustained a potentially mutagenic impact and, therefore, may contribute to acquisition of mutations in other genes.
Metabolism
[edit]Akt2 is required for the insulin-induced translocation of glucose transporter 4 (GLUT4) to theplasma membrane.Glycogen synthase kinase 3 (GSK-3) could be inhibited upon phosphorylation by Akt, which results in increase of glycogen synthesis. GSK3 is also involved inWntsignaling cascade, so Akt might be also implicated in the Wnt pathway. Its role inHCVinducedsteatosisis unknown.[citation needed]
Lysosomal biogenesis and autophagy
[edit]Akt1 regulatesTFEB,a master controller of lysosomal biogenesis,[26]by direct phosphorylation at serine 467.[27]Phosphorylated TFEB is excluded from the nucleus and less active.[27]Pharmacological inhibition of Akt promotes nuclear translocation ofTFEB,lysosomal biogenesis and autophagy.[27]
Angiogenesis
[edit]Akt1 has also been implicated inangiogenesisand tumor development. Although deficiency of Akt1 in mice inhibited physiological angiogenesis, it enhanced pathological angiogenesis and tumor growth associated with matrix abnormalities in skin and blood vessels.[28][29]
Clinical relevance
[edit]Akt proteins are associated with tumor cell survival, proliferation, and invasiveness. The activation of Akt is also one of the most frequent alterations observed in human cancer and tumor cells. Tumor cells that have constantly active Akt may depend on Akt for survival.[30]Therefore, understanding the Akt proteins and their pathways is important for the creation of better therapies to treat cancer and tumor cells. A mosaic-activating mutation (c. 49G→A, p.Glu17Lys) in Akt1 is associated with the Proteus Syndrome, which causes overgrowth of skin, connective tissue, brain and other tissues.[31]
Akt inhibitors
[edit]Akt inhibitors may treat cancers such asneuroblastoma.Some Akt inhibitors have undergone clinical trials. In 2007VQD-002had a phase I trial.[32] In 2010Perifosinereached phase II.[33]but it failed phase III in 2012.
Miltefosineis approved forleishmaniasisand under investigation for other indications including HIV.
Akt1 is now thought to be the "key" for cell entry byHSV-1andHSV-2(herpes virus: oral and genital, respectively). Intracellularcalciumrelease by the cell allows for entry by the herpes virus; the virus activates Akt1, which in turn causes the release of calcium. Treating the cells with Akt inhibitors before virus exposure leads to a significantly lower rate of infection.[34]
MK-2206reported phase 1 results for advanced solid tumors in 2011,[35]and subsequently has undergone numerous phase II studies for a wide variety of cancer types.[36]
In 2013AZD5363reported phase I results regarding solid tumors.[37]with a study of AZD5363 witholaparibreporting in 2016.[38]
Ipatasertibis in phase II trials for breast cancer.[39]
Decreased Akt isoforms can cause deleterious effects
[edit]Akt isoform activation is associated with many malignancies; however, a research group fromMassachusetts General HospitalandHarvard Universityunexpectedly observed a converse role for Akt and one of its downstream effectorFOXOsinacute myeloid leukemia(AML). They claimed that low levels of Akt activity associated with elevated levels of FOXOs are required to maintain the function and immature state ofleukemia-initiating cells(LICs). FOXOs are active, implying reduced Akt activity, in ~40% of AML patient samples regardless of genetic subtype; and either activation of Akt or compound deletion of FoxO1/3/4 reduced leukemic cell growth in a mouse model.[40]
Hyperactivation of Akt1 can cause deleterious effects
[edit]Two studies show that Akt1 is involved in Juvenile Granulosa Cell tumors (JGCT). In-frame duplications in the pleckstrin-homology domain (PHD) of the protein were found in more than 60% of JGCTs occurring in girls under 15 years of age. The JGCTs without duplications carried point mutations affecting highly conserved residues. The mutated proteins carrying the duplications displayed a non-wild-type subcellular distribution, with a marked enrichment at the plasma membrane. This led to a striking degree of Akt1 activation demonstrated by a strong phosphorylation level and corroborated by reporter assays.[41]
Analysis by RNA-Seq pinpointed a series of differentially expressed genes, involved in cytokine and hormone signaling and cell division-related processes. Further analyses pointed to a possible dedifferentiation process and suggested that most of the transcriptomic dysregulations might be mediated by a limited set of transcription factors perturbed by Akt1 activation. These results incriminate somatic mutations of Akt1 as major probably driver events in the pathogenesis of JGCTs.[42]
See also
[edit]- Akt/PKB signaling pathway
- Discovery and development of mTOR inhibitors
- PI3K/AKT/mTOR pathway
- Akt inhibitor
- PTEN
References
[edit]- ^PDB:3MV5;Freeman-Cook KD, Autry C, Borzillo G, Gordon D, Barbacci-Tobin E, Bernardo V, et al. (June 2010). "Design of selective, ATP-competitive inhibitors of Akt".Journal of Medicinal Chemistry.53(12): 4615–22.doi:10.1021/jm1003842.PMID20481595.
- ^PDB:3D0E;Heerding DA, Rhodes N, Leber JD, Clark TJ, Keenan RM, Lafrance LV, et al. (September 2008)."Identification of 4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-{[(3S)-3-piperidinylmethyl]oxy}-1H-imidazo[4,5-c]pyridin-4-yl)-2-methyl-3-butyn-2-ol (GSK690693), a novel inhibitor of AKT kinase".Journal of Medicinal Chemistry.51(18): 5663–79.doi:10.1021/jm8004527.PMID18800763.
- ^Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, et al. (September 2001)."Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene".Genes & Development.15(17): 2203–8.doi:10.1101/gad.913901.PMC312770.PMID11544177.
- ^Nitulescu GM, Van De Venter M, Nitulescu G, Ungurianu A, Juzenas P, Peng Q, et al. (December 2018)."The Akt pathway in oncology therapy and beyond (Review)".International Journal of Oncology.53(6): 2319–2331.doi:10.3892/ijo.2018.4597.PMC6203150.PMID30334567.
- ^abcPal DS, Banerjee T, Lin Y, de Trogoff F, Borleis J, Iglesias PA, et al. (July 2023)."Actuation of single downstream nodes in growth factor network steers immune cell migration".Developmental Cell.58(13): 1170–1188.e7.doi:10.1016/j.devcel.2023.04.019.PMC10524337.PMID37220748.
- ^Staal SP, Hartley JW, Rowe WP (July 1977)."Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma".Proceedings of the National Academy of Sciences of the United States of America.74(7): 3065–7.Bibcode:1977PNAS...74.3065S.doi:10.1073/pnas.74.7.3065.PMC431413.PMID197531.
- ^Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, et al. (July 2003)."Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta".The Journal of Clinical Investigation.112(2): 197–208.doi:10.1172/JCI16885.PMC164287.PMID12843127.
- ^Yang ZZ, Tschopp O, Baudry A, Dümmler B, Hynx D, Hemmings BA (April 2004). "Physiological functions of protein kinase B/Akt".Biochemical Society Transactions.32(Pt 2): 350–4.doi:10.1042/BST0320350.PMID15046607.
- ^Hill MM, Hemmings BA (2002). "Inhibition of protein kinase B/Akt. implications for cancer therapy".Pharmacology & Therapeutics.93(2–3): 243–51.doi:10.1016/S0163-7258(02)00193-6.PMID12191616.
- ^Mitsiades CS, Mitsiades N, Koutsilieris M (May 2004). "The Akt pathway: molecular targets for anti-cancer drug development".Current Cancer Drug Targets.4(3): 235–56.doi:10.2174/1568009043333032.PMID15134532.
- ^Xie J, Weiskirchen R (2020)."What Does the" AKT "Stand for in the Name" AKT Kinase "? Some Historical Comments".Frontiers in Oncology.10:1329.doi:10.3389/fonc.2020.01329.PMC7431881.PMID32850422.
- ^Franke TF, Kaplan DR, Cantley LC, Toker A (January 1997). "Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate".Science.275(5300): 665–8.doi:10.1126/science.275.5300.665.PMID9005852.S2CID31186873.
- ^Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (February 2005). "Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex".Science.307(5712): 1098–101.Bibcode:2005Sci...307.1098S.doi:10.1126/science.1106148.PMID15718470.S2CID45837814.
- ^Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. (October 2006)."SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity".Cell.127(1): 125–37.doi:10.1016/j.cell.2006.08.033.PMID16962653.S2CID230319.
- ^abMahajan K, Coppola D, Challa S, Fang B, Chen YA, Zhu W, et al. (March 2010)."Ack1 mediated AKT/PKB tyrosine 176 phosphorylation regulates its activation".PLOS ONE.5(3): e9646.Bibcode:2010PLoSO...5.9646M.doi:10.1371/journal.pone.0009646.PMC2841635.PMID20333297.
- ^Stuenaes JT, Bolling A, Ingvaldsen A, Rommundstad C, Sudar E, Lin FC, et al. (May 2010)."Beta-adrenoceptor stimulation potentiates insulin-stimulated PKB phosphorylation in rat cardiomyocytes via cAMP and PKA".British Journal of Pharmacology.160(1): 116–29.doi:10.1111/j.1476-5381.2010.00677.x.PMC2860212.PMID20412069.
- ^Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, et al. (February 2008). "Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance".Nature.451(7181): 964–9.Bibcode:2008Natur.451..964Y.doi:10.1038/nature06668.PMID18288188.S2CID18459576.
- ^Fan CD, Lum MA, Xu C, Black JD, Wang X (January 2013)."Ubiquitin-dependent regulation of phospho-AKT dynamics by the ubiquitin E3 ligase, NEDD4-1, in the insulin-like growth factor-1 response".The Journal of Biological Chemistry.288(3): 1674–84.doi:10.1074/jbc.M112.416339.PMC3548477.PMID23195959.
- ^Cooper GM (2000)."Figure 15.37: PTEN and PI3K".The cell: a molecular approach.Washington, D.C: ASM Press.ISBN978-0-87893-106-4.
- ^Song G, Ouyang G, Bao S (2005)."The activation of Akt/PKB signaling pathway and cell survival".Journal of Cellular and Molecular Medicine.9(1): 59–71.doi:10.1111/j.1582-4934.2005.tb00337.x.PMC6741304.PMID15784165.
- ^Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002)."Figure 15-60: BAD phosphorylation by Akt".Molecular biology of the cell.New York: Garland Science.ISBN978-0-8153-3218-3.
- ^Lodish H, Berk A, Zipursky LS, Matsudaira P, Baltimore D, Darnell J (1999)."Figure 23-50: BAD interaction with Bcl-2".Molecular cell biology.New York: Scientific American Books.ISBN978-0-7167-3136-8.
- ^Faissner A, Heck N, Dobbertin A, Garwood J (2006). "DSD-1-Proteoglycan/Phosphacan and Receptor Protein Tyrosine Phosphatase-Beta Isoforms during Development and Regeneration of Neural Tissues".Brain Repair.Advances in Experimental Medicine and Biology. Vol. 557. pp. 25–53, Figure 2: regulation of NF–κB.doi:10.1007/0-387-30128-3_3.ISBN978-0-306-47859-8.PMID16955703.
- ^Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, et al. (March 1999)."Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway".Proceedings of the National Academy of Sciences of the United States of America.96(5): 2110–5.Bibcode:1999PNAS...96.2110R.doi:10.1073/pnas.96.5.2110.PMC26745.PMID10051603.
- ^Kandel ES, Skeen J, Majewski N, Di Cristofano A, Pandolfi PP, Feliciano CS, et al. (November 2002)."Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage".Molecular and Cellular Biology.22(22): 7831–41.doi:10.1128/MCB.22.22.7831-7841.2002.PMC134727.PMID12391152.
- ^Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, et al. (July 2009)."A gene network regulating lysosomal biogenesis and function".Science.325(5939): 473–7.Bibcode:2009Sci...325..473S.doi:10.1126/science.1174447.PMID19556463.S2CID20353685.
- ^abcPalmieri M, Pal R, Nelvagal HR, Lotfi P, Stinnett GR, Seymour ML, et al. (February 2017)."mTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases".Nature Communications.8:14338.Bibcode:2017NatCo...814338P.doi:10.1038/ncomms14338.PMC5303831.PMID28165011.
- ^Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, et al. (November 2005)."Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo".Nature Medicine.11(11): 1188–96.doi:10.1038/nm1307.PMC2277080.PMID16227992.
- ^Somanath PR, Razorenova OV, Chen J, Byzova TV (March 2006)."Akt1 in endothelial cell and angiogenesis".Cell Cycle.5(5): 512–8.doi:10.4161/cc.5.5.2538.PMC1569947.PMID16552185.
- ^"Tumor Genetics; AKT Function and Oncogenic Activity"(PDF).Scientific Report.Fox Chase Cancer Center. 2005. Archived fromthe original(PDF)on 2010-06-04.Retrieved2013-01-23.
- ^Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, et al. (August 2011)."A mosaic activating mutation in AKT1 associated with the Proteus syndrome".The New England Journal of Medicine.365(7): 611–9.doi:10.1056/NEJMoa1104017.PMC3170413.PMID21793738.
- ^"VioQuest Pharmaceuticals Announces Phase I/IIa Trial For Akt Inhibitor VQD-002".Apr 2007.
- ^Ghobrial IM, Roccaro A, Hong F, Weller E, Rubin N, Leduc R, et al. (February 2010)."Clinical and translational studies of a phase II trial of the novel oral Akt inhibitor perifosine in relapsed or relapsed/refractory Waldenstrom's macroglobulinemia".Clinical Cancer Research.16(3): 1033–41.doi:10.1158/1078-0432.CCR-09-1837.PMC2885252.PMID20103671.
- ^Cheshenko N, Trepanier JB, Stefanidou M, Buckley N, Gonzalez P, Jacobs W, et al. (July 2013)."HSV activates Akt to trigger calcium release and promote viral entry: novel candidate target for treatment and suppression".FASEB Journal.27(7): 2584–99.doi:10.1096/fj.12-220285.PMC3688744.PMID23507869.
- "Scientists Reveal Novel Strategy for Stopping Herpes".Science News.Apr 5, 2013.
- ^Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. (December 2011). "First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors".Journal of Clinical Oncology.29(35): 4688–95.doi:10.1200/JCO.2011.35.5263.PMID22025163.
- ^MK-2206 phase-2 trials
- ^AKT inhibitor AZD5363 well tolerated, yielded partial response in patients with advanced solid tumors
- ^"PARP/AKT Inhibitor Combination Active in Multiple Tumor Types. April 2016".Archived fromthe originalon 2016-05-07.Retrieved2016-04-20.
- ^Jabbarzadeh Kaboli P, Salimian F, Aghapour S, Xiang S, Zhao Q, Li M, et al. (June 2020). "Akt-targeted therapy as a promising strategy to overcome drug resistance in breast cancer - A comprehensive review from chemotherapy to immunotherapy".Pharmacological Research.156:104806.doi:10.1016/j.phrs.2020.104806.PMID32294525.S2CID215793444.
- ^Sykes SM, Lane SW, Bullinger L, Kalaitzidis D, Yusuf R, Saez B, et al. (September 2011)."AKT/FOXO signaling enforces reversible differentiation blockade in myeloid leukemias".Cell.146(5): 697–708.doi:10.1016/j.cell.2011.07.032.PMC3826540.PMID21884932.
- ^Bessière L, Todeschini AL, Auguste A, Sarnacki S, Flatters D, Legois B, et al. (May 2015)."A Hot-spot of In-frame Duplications Activates the Oncoprotein AKT1 in Juvenile Granulosa Cell Tumors".eBioMedicine.2(5): 421–31.doi:10.1016/j.ebiom.2015.03.002.PMC4485906.PMID26137586.
- ^Auguste A, Bessière L, Todeschini AL, Caburet S, Sarnacki S, Prat J, et al. (December 2015). "Molecular analyses of juvenile granulosa cell tumors bearing AKT1 mutations provide insights into tumor biology and therapeutic leads".Human Molecular Genetics.24(23): 6687–98.doi:10.1093/hmg/ddv373.PMID26362254.
Further reading
[edit]- Los M, Maddika S, Erb B, Schulze-Osthoff K (May 2009)."Switching Akt: from survival signaling to deadly response".BioEssays.31(5): 492–5.doi:10.1002/bies.200900005.PMC2954189.PMID19319914.
- Quaresma AJ, Sievert R, Nickerson JA (April 2013)."Regulation of mRNA export by the PI3 kinase/AKT signal transduction pathway".Molecular Biology of the Cell.24(8): 1208–21.doi:10.1091/mbc.E12-06-0450.PMC3623641.PMID23427269.
External links
[edit]- Proto-Oncogene+Proteins+c-aktat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
