Paclitaxel trevatide
 | |
| Clinical data | |
|---|---|
| Other names | NG1005; GRN1005 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
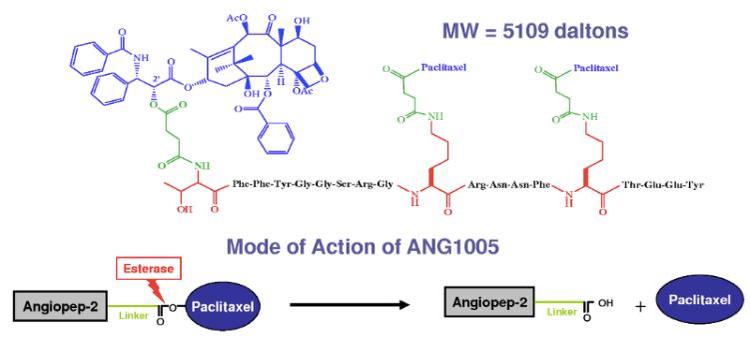
| Formula | C257H308N32O79 |
| Molar mass | 5109.436g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Paclitaxel trevatide(development codesNG1005andGRN1005) is an experimental chemotherapy drug that is under development by Angiochem Inc, a Canadian biotech company. Phase II clinical trials have completed for several indications, and the company is preparing for phase III trials.[1]
Paclitaxel trevatide is apaclitaxel-Angiopep-2 conjugate. Various Angiopep vectors have been composed and differ by their anti-cancer moieties. This has then been shown to be a prospective cancer therapy drug that can not only be conjugated to paclitaxel but also peptides, monoclonal antibodies, siRNA and many other biological materials. Paclitaxel trevatide has the potential to treat a variety ofCNSdiseases including glioma.[2]Research has shown reduction in tumor growth in mice and rats withglioblastoma.[3][4][5]
Mechanism of action
[edit]This drug was specifically designed to treat braintumors.Because of theblood brain barrier(BBB), many cancer therapy drugs are prevented from passing through the braincapillariesinto the parenchyma.[6]Paclitaxel is generally prevented from reaching its target in the cell due to the presence of the efflux pumpP-glycoprotein(P-gp) at the barrier. This is known as amultidrug resistant-associated protein(MRP1) that causes resistance amongst many organic drugs that are not conjugated to acidic ligands.[7]This receptor is an ATP-driven transporter that will pump drugs, drug metabolites, and endogenous metabolites out of the cell.[citation needed]
Paclitaxel trevatide contains paclitaxel, which stabilizesmicrotubulepolymer formation. Microtubules are composed of polymers consisting of the protein tubulin. Therefore, paclitaxel binds at the site of β-tubulin and induces polymerization, thus protecting the microtubule from disassembling during mitosis. This blocks the progression of mitosis due to a prolonged activation of the microtubule in themitotic checkpoint,resulting in cellapoptosisor reversion to theG0 phase.However, paclitaxel trevatide will effectively transport across the BBB with approximately a 100-fold higher transport rate compared to a free paclitaxel.[8] Paclitaxel trevatide will cross the capillary medium via receptor mediated transcytosis of the low-densitylipoprotein receptor-related protein1 (LRP1) which is upregulated in some cancers.[9]Ester hydrolyzing enzymes (esterases) then catalyze a highly stereospecific reaction that results inhydrolysisof the paclitaxel trevatide ester tocarboxylic acids.This results in the intracellular release of paclitaxel and subsequent action on tubulin.[citation needed]
Concentration has been found to play a key role paclitaxel trevatide's administration. Studies show that a high concentration will suppress microtubule detachment which is necessary for mitosis to occur.[10]The microenvironment of cells may also differ from that of rapidly proliferating cancer cells. This is generally more oxygen deficient and acidic. These conditions will then result in a selective pressure towards cancer cellproliferation.It has been shown that cancer cell lines exposed to this hypoxic or serum-deprived condition will upregulate LRP1 expression, thus leading to an increased uptake of paclitaxel trevatide.[11]However, these receptors are also present in non-cancerous cells, thus presenting a challenge due to non-specific targeting. It is then believed that because these receptors are overexposed in tumors, that receptor binding may be in favor of the cancer cells.[citation needed]
Clinical trials
[edit]In 2008, two phase I clinical trials of paclitaxel trevatide were started; one in patients with advanced cancer and brain metastases,[12]and another in patients with recurrent malignantglioma.[13]Favorable initial tolerability results in brain cancer were reported in March 2009.[14]and more in October 2009 and updated in June 2010.[9] In 2014, paclitaxel trevatide received orphan drug designation from FDA for the treatment of glioblastoma multiform[15] As of January 2016, Angiochem has conducted phase II trials for the treatment of breast cancer with brain metastasis, glioblastoma, and high-grade glioma.[16]
Results from the phase II trial in patients with breast cancer and leptomeningeal carcinomatosis, a fatal form of brain metastasis, were presented atASCOin 2016.[17]
References
[edit]- ^"Angiochem Announces Successful End-of-Phase 2 Meeting with FDA for ANG1005 | Angiochem: Peptide-Antibody Conjugates that Cross the Blood Brain Barrier".angiochem.com.
- ^"ANG1005 - A Promising New Targeted Taxane Derivative".Archived fromthe originalon 27 May 2013.Retrieved5 June2013.
- ^Régina A, Demeule M, Ché C, Lavallée I, Poirier J, Gabathuler R, Béliveau R, Castaigne JP (2008)."Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2".Br J Pharmacol.155(2): 185–197.doi:10.1038/bjp.2008.260.PMC2538693.PMID18574456.
- ^Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA, Mittapalli RK, Palmieri D, Steeg PS, Lockman PR, Smith QR (November 2009)."Uptake of ANG1005, a Novel Paclitaxel Derivative, Through the Blood-Brain Barrier into Brain and Experimental Brain Metastases of Breast Cancer".Pharm Res.26(11): 2486–94.doi:10.1007/s11095-009-9964-5.PMC2896053.PMID19774344.
- ^Patel MM, Goyal BR, Bhadada SV, Bhatt JS, Amin AF (2009). "Getting into the brain: approaches to enhance brain drug delivery".CNS Drugs.23(1): 35–58.doi:10.2165/0023210-200923010-00003.PMID19062774.S2CID26113811.
- ^Thomas FC, Taskar K, Rudraraju V, et al. (November 2009)."Uptake of ANG1005, a Novel Paclitaxel Derivative, Through the Blood-Brain Barrier into Brain and Experimental Brain Metastases of Breast Cancer".Pharm. Res.26(11): 2486–94.doi:10.1007/s11095-009-9964-5.PMC2896053.PMID19774344.
- ^Shao K, Huang R, Li J, Han L, Ye L, Lou J, Jiang C (2010). "Angiopep-2 modified PE-PEG based polymeric micelles for amphotericin B delivery targeted to the brain".J Control Release.147(1): 118–26.doi:10.1016/j.jconrel.2010.06.018.PMID20609375.
- ^"Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1"(PDF).
- ^ab"Angiochem Presents Complete Phase 1 /2 Clinical Data for ANG1005: Further Demonstrating Benefits of Targeting LRP-1 Pathway in Cancer".Pharmalive.com. Archived fromthe originalon 2011-07-15.Retrieved2010-06-08.(paid subscription required)[unreliable medical source?][verification needed]
- ^Régina A, Demeule M, Ché C, et al. (September 2008)."Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2".Br. J. Pharmacol.155(2). British Journal of Pharmacology: 185–97.doi:10.1038/bjp.2008.260.PMC2538693.PMID18574456.
- ^"nfluence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein"(PDF).British Journal of Cancer.
- ^"A Phase 1, Open-Label, Dose Escalation Study of ANG1005 in Patients With Advanced Solid Tumors and Metastatic Brain Cancer".3 June 2010.Retrieved16 August2010.
- ^"angiochems-ang1005-demonstrates-preliminary-clinical-safety-and-tolerability-in-brain-cancers"(Press release). CheckOrphan.com. Halsin Partners. Archived fromthe originalon 22 July 2012.Retrieved16 August2010.[unreliable medical source?]
- ^"AngioChem's ANG1005 Shows Promise In The Treatment Of Brain Cancers".23 October 2008.
- ^"Angiochem's ANG1005 Received Orphan Drug Designation from FDA for the Treatment of Glioblastoma multiform | Angiochem: Peptide-Antibody Conjugates that Cross the Blood Brain Barrier".angiochem.com.
- ^"ANG1005 in Patients With Recurrent High-Grade Glioma".clinicaltrials.gov.
- ^"Angiochem's ANG1005 Shows Clinical Benefit and Prolonged Survival in Phase II Trial | Angiochem: Peptide-Antibody Conjugates that Cross the Blood Brain Barrier".angiochem.com.
External links
[edit]- Mazza M, Uchegbu IF, Schätzlein AG (September 2008)."Cancer and the blood–brain barrier: 'Trojan horses' for courses?".Br J Pharmacol.155(2): 149–51.doi:10.1038/bjp.2008.274.PMC2538701.PMID18587417.
- "ANG1005 Crosses The Blood-Brain Barrier To Reduce Tumor Size And Is Effective In Resistant Tumors".Medical News Today.
- "AngioChem's ANG1005 demonstrates preliminary clinical safety and tolerability in brain cancers"(Press release). Eurekalert. Halsin Partners. October 2008.