Acivicin
| |
| Names | |
|---|---|
| IUPAC name
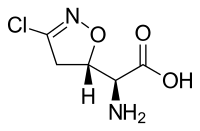
(2S)-Amino[(5S)-3-chloro-4,5-dihydro-1,2-oxazol-5-yl]ethanoic acid
| |
| Other names
Antibiotic AT 125
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C5H7ClN2O3 | |
| Molar mass | 178.574 |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Acivicinis an analog ofglutamine.It is aninhibitorofgamma-glutamyl transferase.
It is afermentationproduct ofStreptomyces sviceus.[1]It interferes with glutamate metabolism and inhibits glutamate dependent synthesis ofenzymes,and is thereby potentially helpful in treatment ofsolid tumors.[2]
After its discovery in 1972, acivicin was studied as an anti-cancer agent, but trials were unsuccessful due to toxicity.[3]
Research[edit]
An in vitro study showed that Acivicin at a concentration of 5 μM Acivicin inhibited by 78% the growth of humanpancreaticcarcinomacells(MIA PaCa-2) after 72 hours in continuous culture. It was also found that acivicin at a concentration of 450 μM irreversibly inactivated MIA PaCa-2 γ-glutamyl transpeptidase (10 nmol/min/106cells) with an inactivation half-life of 80 minutes.[1]
Phase I studies[edit]
Phase I dose escalating studies conducted in 23 cancer patients administered acivicin with a concomitant 96-h i.v. infusion of a mixture of 16 amino acids showed reversible, dose-limiting CNS toxicity, characterized by lethargy, confusion and decreased mental status.
References[edit]
- ^abAllen, L.; Meck, R.; Yunis, A. (1980). "The Inhibition of γ-Glutamyl Transpeptidase from Human Pancreatic Carcinoma Cells by (αS,5S)-α-Amino-3-chloro-4,5-dihydro-5-isoxazoleacetic Acid (AT-125; NSC-163501)".Research Communications in Chemical Pathology and Pharmacology.27(1): 175–182.PMID6102405.
- ^Hidalgo, M.; Rodriguez, G.; Kuhn, J. G.; Brown, T.; Weiss, G.; MacGovren, J. P.; von Hoff, D. D.; Rowinsky, E. K. (1998). "A Phase I and Pharmacological Study of the Glutamine Antagonist Acivicin with the Amino Acid Solution Aminosyn in Patients with Advanced Solid Malignancies".Clinical Cancer Research.4(11): 2763–2770.PMID9829740.
- ^Kreuzer, Johannes; Bach, Nina C.; Forler, Daniel; Sieber, Stephan A. (2015)."Target discovery of acivicin in cancer cells elucidates its mechanism of growth inhibition".Chemical Science.6(1): 237–245.doi:10.1039/C4SC02339K.PMC4285139.PMID25580214.
External links[edit]
- Obrador, E.; Carretero, J.; Ortega, A.; Medina, I.; Rodilla, V.; Pellicer, J. A.; Estrela, J. M. (2002)."γ-Glutamyl Transpeptidase Overexpression Increases Metastatic Growth of B16 Melanoma Cells in the Mouse Liver".Hepatology.35(1): 74–81.doi:10.1053/jhep.2002.30277.PMID11786961.
- Schmees, C.; Prinz, C.; Treptau, T.; Rad, R.; Hengst, L.; Voland, P.; Bauer, S.; Brenner, L.; Schmid, R. M.; Gerhard, M. (2007)."Inhibition of T-Cell Proliferation byHelicobacter pyloriγ-Glutamyl Transpeptidase ".Gastroenterology.132(5): 1820–1833.doi:10.1053/j.gastro.2007.02.031.PMID17484877.
