Adenoviridae
| Adenoviruses | |
|---|---|

| |
| Transmission electron micrographof two adenovirus particles | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Varidnaviria |
| Kingdom: | Bamfordvirae |
| Phylum: | Preplasmiviricota |
| Class: | Tectiliviricetes |
| Order: | Rowavirales |
| Family: | Adenoviridae |
| Genera | |
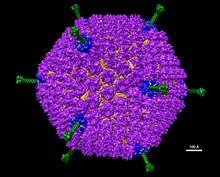
Adenoviruses(members of thefamilyAdenoviridae) are medium-sized (90–100nm),nonenveloped(without an outer lipid bilayer)viruseswith anicosahedralnucleocapsidcontaining adouble-stranded DNAgenome.[2]Their name derives from their initial isolation from humanadenoidsin 1953.[3]
They have a broad range ofvertebratehosts; in humans, more than 50 distinct adenoviralserotypeshave been found to cause a wide range ofillnesses,from mild respiratory infections in young children (known as thecommon cold) to life-threatening multi-organ disease in people with aweakened immune system.[2]
Virology
[edit]Classification
[edit]This family contains the followinggenera:[4]
- Atadenovirus
- Aviadenovirus
- Ichtadenovirus
- Mastadenovirus(including all human adenoviruses)
- Siadenovirus
- Testadenovirus
Diversity
[edit]In humans, currently there are 88 human adenoviruses (HAdVs) in seven species (Human adenovirus A to G):[5]
- A:12, 18, 31
- B:3, 7, 11,14,16, 21, 34, 35, 50, 55
- C:1, 2, 5, 6, 57[6]
- D:8, 9, 10, 13, 15, 17, 19, 20, 22, 23, 24, 25, 26, 27, 28, 29, 30, 32, 33,36,37, 38, 39, 42, 43, 44, 45, 46, 47, 48, 49, 51, 53, 54, 56,[7]58, 59, 60, 62, 63,[8]64, 65, 67, 69,[9]70, 71, 72, 73, 74, 75
- E:4
- F:40,41[10]
- G:52[11]
Different types/serotypes are associated with different conditions:[12]
- respiratory disease(mainly species HAdV-B and C)
- conjunctivitis(HAdV-B and D)
- gastroenteritis(HAdV-F types 40, 41, HAdV-G type 52)
- obesityor adipogenesis (HAdV-A type 31, HAdV-C type 5, HAdV-D types 9, 36, 37)[13]
All these types are called Human mastadenovirus A–G by theICTV,because all are members of the genusMastadenovirus.
Structure
[edit]
Adenoviruses are medium-sized (90–100 nm).[2]Thevirionsare composed of one linear piece ofdouble-stranded DNAinside an icosahedralcapsid.240hexon proteinsmake up the bulk of the capsid, while twelve penton bases cap the icosahedron's corners. The penton bases are associated with protruding fibers that aid inattachmentto thehostcellvia the receptor on its surface.[14]
In 2010, the structure of the human adenovirus was solved at the atomic level, making it the largest high-resolution model ever. The virus is composed of around 1 millionamino acidresidues and weighs around 150MDa.[15][16]
Genome
[edit]
The adenovirus genome is linear, non-segmented double-stranded (ds) DNA that is between 26 and 48Kbp.[2]This allows the virus to theoretically carry 22 to 40genes.Although this is significantly larger than other viruses in itsBaltimore group,it is still a very simple virus and is heavily reliant on the host cell for survival and replication. The genes of adenoviruses can generally be divided into well-conserved sets of transcription units with six early transcription units (E1A, E1B, E2A, E2B, E3 and E4) and one late transcription unit ranging from L1-L5. In addition, adenoviruses also contain two intermediate transcription units named XI and IVa2. To increase the viral gene economy, adenoviruses accommodate genes on both strands of its dsDNA meaning that most of its genome is utilized for coding proteins.[17]An interesting feature of this viral genome is that it has a terminal 55kDaprotein associated with each of the 5' ends of the linear dsDNA. These are used as primers in viral replication and ensure that the ends of the virus' linear genome are adequately replicated.[citation needed]
Replication
[edit]Adenoviruses possess a linear dsDNAgenomeand are able toreplicatein thenucleusofvertebratecells using the host's replication machinery.[2]Entry of adenoviruses into the host cell involves two sets of interactions between the virus and the host cell.[2]Most of the action occurs at the vertices. Entry into the host cell is initiated by the knobdomainof the fiber protein binding to the cell receptor.[2]The two currently established receptors are:CD46for the group B human adenovirus serotypes and thecoxsackievirus/adenovirus receptor (CAR)for all other serotypes.[2]There are some reports suggestingMHCmolecules andsialic acidresidues functioning in this capacity as well. This is followed by a secondary interaction, where a motif in the penton base protein (seecapsomere) interacts with anintegrinmolecule. It is the co-receptor interaction that stimulates entry of the adenovirus. This co-receptor molecule isαV integrin.Binding to αv integrin results inendocytosisof the virus particle viaclathrin-coatedpits. Attachment to αV integrin stimulates cell signaling and thus inducesactinpolymerization, which facilitates clathrin-mediated endocytosis, and results in virion's entry into the host cell within anendosome.[18]
Once the virus has successfully gained entry into the host cell, the endosome acidifies, which alters virus topology by causing capsid components to disband. The capsid is destabilized and protein VI, which is one of the capsid constituents (seeAdenovirus genome) is released from it.[19]Protein VI contains an N-terminal amphiphatic alpha-helix, a helical domain that exhibits both hydrophobic and hydrophilic properties. This amphipathic helix enables the binding of protein VI to the endsomal membrane leading to a severe membrane curvature that ultimately disrupts the endosome.[20]These changes, as well as the toxic nature of the pentons, destroy the endosome, resulting in the movement of the virion into the cytoplasm.[2]With the help of cellularmicrotubules,the virus is transported to the nuclear pore complex, whereby the adenovirus particle disassembles. Viral DNA is subsequently released, which can enter thenucleusvia thenuclear pore.[21]After this the DNA associates withhistonemolecules already present in the nucleus, which allows it to interact with the host cell transcription machinery.[22]Then, viral gene expression can occur, without integrating the viral genome into host cell chromosomes,[23]and new virus particles can be generated.
The adenoviruslife cycleis separated by theDNA replicationprocess into two phases: an early and a late phase.[2]In both phases, aprimary transcriptthat isalternatively splicedto generatemonocistronic mRNAscompatible with the host'sribosomeis generated, allowing for the products to betranslated.[citation needed]
The early genes are responsible for expressing mainly non-structural, regulatoryproteins.[2]The goal of these proteins is threefold: to alter the expression of host proteins that are necessary forDNA synthesis;to activate other virus genes (such as the virus-encodedDNA polymerase); and to avoid premature death of the infected cell by the host-immune defenses (blockage ofapoptosis,blockage ofinterferonactivity, and blockage ofMHC class Itranslocation and expression).
Some adenoviruses under specialized conditions can transform cells using their early gene products.E1A(bindsRetinoblastoma tumor suppressor protein) has been found to immortalize primary cellsin vitroallowing E1B (bindsp53tumor suppressor) to assist and stably transform the cells. Nevertheless, they are reliant upon each other to successfully transform the host cell and formtumors.E1A is mostly intrinsically disordered protein and contains CR3 domain which is critical for transcriptional activation.[24]
DNA replication separates the early and late phases. Once the early genes have liberated adequate virus proteins, replication machinery, and replication substrates, replication of the adenovirus genome can occur. A terminal protein that is covalently bound to the 5' end of the adenovirus genome acts as aprimerfor replication. The viral DNA polymerase then uses a strand displacement mechanism, as opposed to the conventionalOkazaki fragmentsused in mammalian DNA replication, to replicate the genome.
The late phase of the adenovirus lifecycle is focused on producing sufficient quantities of structural protein to pack all the genetic material produced by DNA replication.[2]Once the viral components have successfully been replicated, the virus is assembled into its protein shells and released from the cell as a result of virally induced celllysis.[2]
Multiplicity reactivation
[edit]Adenovirus is capable of multiplicity reactivation (MR)[25](Yamamoto and Shimojo, 1971). MR is the process by which two, or more, virus genomes containing lethal damage interact within the infected cell to form a viable virus genome. Such MR was demonstrated for adenovirus 12 after virions were irradiated with UV light and allowed to undergo multiple infection of host cells.[25]In a review, numerous examples of MR in different viruses were described, and it was suggested that MR is a common form of sexual interaction that provides the survival advantage of recombinational repair of genome damages.[26]
Epidemiology
[edit]Transmission
[edit]Adenoviruses are unusually stable tochemicalor physical agents and adversepHconditions, allowing for prolonged survival outside of the body and water. Adenoviruses are spread primarily via respiratory droplets, however they can also be spread byfecalroutes and via aerosols (airborne transmission).[27]Research into the molecular mechanisms underlying adenoviral transmission provide empirical evidence in support of the hypothesis thatcoxsackievirus/adenovirus receptors (CARs)are needed to transport adenoviruses into certain naive/progenitor cell types.[28]
Humans
[edit]Humans infected with adenoviruses display a wide range of responses, from no symptoms at all to the severe infections typical ofAdenovirus serotype 14.
Animals
[edit]Bat adenovirus TJM(Bt-AdV-TJM) is a novel species of theMastadenovirusgenus isolated fromMyotisandScotophilus kuhliiin China.[29]It is most closely related to thetree shrewand canine AdVs.[30]
Two types ofcanineadenoviruses are well known, type 1 and 2. Type 1 (CAdV-1) causesinfectious canine hepatitis,a potentially fatal disease involvingvasculitisandhepatitis.Type 1 infection can also cause respiratory and eye infections. CAdV-1 also affects foxes (Vulpes vulpesandVulpes lagopus) and may cause hepatitis and encephalitis.Canine adenovirus 2(CAdV-2) is one of the potential causes ofkennel cough.Corevaccinesfordogsinclude attenuated live CAdV-2, which produces immunity to CAdV-1 and CAdV-2. CAdV-1 was initially used in a vaccine for dogs, butcornealedemawas a common complication.[31]
Squirrel adenovirus (SqAdV) is reported to cause enteritis in red squirrels in Europe, while gray squirrels seem to be resistant. SqAdV is most closely related to the adenovirus of guinea pigs (GpAdV).
Adenovirus in reptilesis poorly understood, but research is currently in progress.
Adenoviruses are also known to cause respiratory infections inhorses,cattle,pigs,sheep,andgoats.Equine adenovirus 1can also cause fatal disease in immunocompromisedArabian foals,involving pneumonia and destruction ofpancreaticandsalivary glandtissue.[31]Tupaia adenovirus(TAV) (tree shrew adenovirus 1) has been isolated from tree shrews.
Otarine adenovirus 1 has been isolated fromsea lions(Zalophus californianus).[32]
The fowl adenoviruses are associated with many disease conditions in domestic fowl likeinclusion body hepatitis,hydropericardium syndrome,[33]Egg drop syndrome,Quail bronchitis,Gizzard erosionsand many respiratory conditions. They have also been isolated from wildblack kites(Milvus migrans).[34]
Titi monkeyadenovirus was isolated from a colony of monkeys.[35]
Prevention
[edit]Currently there is a vaccine for adenovirus type 4 and 7 for US military personnel only. US military personnel are the recipients of this vaccine because they may be at a higher risk of infection.[citation needed]The vaccine contains a live virus, which may be shed in stool and lead to transmission. The vaccine is not approved for use outside of the military, as it has not been tested in studied in the general population or on people with weakened immune systems.[36]
In the past, US military recruits were vaccinated against two serotypes of adenovirus, with a corresponding decrease in illnesses caused by those serotypes. That vaccine is no longer manufactured. The U.S. Army Medical Research and Materiel Command announced on 31 October 2011 that a new adenovirus vaccine, which replaces the older version that has been out of production for over a decade, was shipped to basic training sites on 18 October 2011. More information is available here.[37]
Prevention of adenovirus, as well as other respiratory illnesses, involves frequent hand washing for more than 20 seconds, avoiding touching the eyes, face, and nose with unwashed hands, and avoiding close contact with people with symptomatic adenovirus infection. Those with symptomatic adenovirus infection are additionally advised to cough or sneeze into the arm or elbow instead of the hand, to avoid sharing cups and eating utensils, and to refrain from kissing others. Chlorination of swimming pools can prevent outbreaks of conjunctivitis caused by adenovirus.[36]
Diagnosis
[edit]Diagnosis is from symptoms and history. Tests are only necessary in very serious cases. Tests include blood tests, eyes, nose or throat swabs, stool sample tests, and chest x-rays.[38]In the laboratory, adenovirus can be identified with antigen detection, polymerase chain reaction (PCR), virus isolation and serology. Even if adenovirus is found to be present, it may not be the cause of any symptoms. Some immunocompromised individuals can shed the virus for weeks and show no symptoms.[39]
Infections
[edit]Most infections with adenovirus result in infections of the upper respiratory tract. Adenovirus infections often present asconjunctivitis,tonsillitis(which may look exactly likestrep throatand cannot be distinguished from strep except by throat culture), anear infection,orcroup.[40]Adenoviruses types 40 and 41 can also causegastroenteritis.[41]A combination of conjunctivitis and tonsillitis is particularly common with adenovirus infections.
Some children (especially the youngest) can develop adenovirusbronchiolitisorpneumonia,both of which can be severe. In babies, adenoviruses can also cause coughing fits that look almost exactly likewhooping cough.Adenoviruses can also causeviral meningitisorencephalitis.Rarely, adenovirus can causehemorrhagic cystitis(inflammation of the urinary bladder—a form ofurinary tract infection—with blood in the urine).
Most people recover from adenovirus infections by themselves, but people withimmunodeficiencysometimes die of adenovirus infections, and—rarely—even previously healthy people can die of these infections.[42]This may be because sometimes adenoviral infection can lead to cardiac disorders. For example, in one study, some cardiac samples of patients with dilated cardiomyopathy were positive for presence of adenovirus type 8.[43]
Adenoviruses are often transmitted by expectoration (e.g. aerosols), but they can also be transmitted by contact with an infected person, or by virus particles left on objects such as towels and faucet handles. Some people with adenovirus gastroenteritis may shed the virus in their stools for months after getting over the symptoms. The virus can be passed through water in swimming pools that are not sufficiently chlorinated.
As with many other illnesses, good handwashing practice is one way to inhibit the person-to-person transmission of adenoviruses. Heat andbleachwill kill adenoviruses on objects.[citation needed]
Treatment
[edit]There are no proven antiviral drugs to treat adenoviral infections, so treatment is largely directed at the symptoms (such asacetaminophenfor fever). The antiviral drugcidofovirhas helped certain of those patients who had severe cases of illness; the number helped and to what degree, and the particular complications or symptoms it helped with, and when and where this happened, were not given in the source.[44]A doctor may give antibiotic eyedrops for conjunctivitis, while awaiting results of bacterial cultures, and to help prevent secondary bacterial infections. Currently, there is no adenovirus vaccine available to the general public, but a vaccine is available for the United States military for Types 4 and 7.
Use in gene therapy and vaccination
[edit]Gene therapy
[edit]Adenoviruses have long been a popularviral vectorforgene therapydue to their ability to affect both replicating and non-replicating cells, accommodate largetransgenes,and code for proteins without integrating genetic material into the host cell genome.[23]More specifically, they are used as a vehicle to administertargeted therapy,[45]in the form ofrecombinant DNAor protein. This therapy has been found especially useful in treatingmonogenicdisease (e.g.cystic fibrosis,X-linkedSCID,alpha1-antitrypsindeficiency) and cancer.[23]In China,oncolytic adenovirusis an approved cancer treatment.[46]Specific modifications onfiber proteinsare used to target Adenovirus to certain cell types;[47]a major effort is made to limithepatotoxicityand prevent multiple organ failure. Adenovirus dodecahedron can qualify as a potent delivery platform for foreign antigens to humanmyeloid dendritic cells(MDC), and that it is efficiently presented by MDC to M1-specific CD8+ T lymphocytes.[48]
A safety issue with adenoviruses is that they can cause an immune response with a related inflammatory response as occurred in the death ofJesse Gelsingerin 1999. To address this risk, the genome of the viral genes have been modified to remove some viral genes. One such modification is thegutless vectorthat removes almost all the viral genome.[49]: 58
Adenovirus has been used for delivery ofCRISPR/Cas9gene editingsystems, but high immune reactivity to viral infection has posed challenges in use for patients.
Vaccines
[edit]Modified (recombinant) adenovirus vectors, including replication incompetent types, can deliver DNA coding for specificantigens.[50]
Adenovirus have been used to produceviral vectorCOVID-19 vaccines."In four candidate COVID-19 vaccines... Ad5... serves as the 'vector' to transport the surface protein gene of SARS-CoV-2".[51]The goal is to genetically express thespike glycoproteinof severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A replication-deficient chimpanzee adenovirus vaccine vector (ChAdOx1) is used by theOxford–AstraZeneca COVID-19 vaccinethat has been approved for use.[52][53]TheJanssen COVID-19 vaccineuses modified recombinant adenovirus type-26 (Ad26).[54]Recombinant adenovirus type-5 (Ad5) are being used byAd5-nCoV,[55]ImmunityBio andUQ-CSL V451.TheGam-COVID-Vac(aka Sputnik-V) product is innovative because an Ad26 based vaccine is used on the first day and an Ad5 vaccine is used on day 21.[54]Another one isChAd-SARS-CoV-2-S;the vaccine reportedly prevented mice that were genetically modified to have humanACE2(hACE2) receptors, presumably receptors that allow virus-entry into the cells, from being infected with SARS-CoV-2.[56][57]
Possible issues with using Adenovirus as vaccine vectors include: the human body develops immunity to the vector itself, making subsequent booster shots difficult or impossible.[58]In some cases, people have pre-existing immunity to Adenoviruses, making vector delivery ineffective.[59]
HIV infection concerns
[edit]This sectionneeds morereliable medical referencesforverificationor relies too heavily onprimary sources.(April 2021) |  |
The use of Ad5 vaccines for COVID-19 worried researchers who had experience with two failed trials of an Ad5 vaccine, Phambili and STEP, due to the increased risk for uncircumcised male patients of contractingHIV-1via unprotected anal sex.[60]At the time, it was concluded that heightened risk of HIV reception may be observed for any Ad5-based vector vaccine.[61]In October 2020, these researchers wrote inThe Lancet:"On the basis of these findings, we are concerned that use of an Ad5 vector for immunisation against SARS-CoV-2 could similarly increase the risk of HIV-1 acquisition among men who receive the vaccine."[62][63]Vaccines using other technologies would not be affected, butSputnik V,ConvideciaandImmunityBio's hAd5would.[64]Two studies found that Ad5-specificCD4T cellsare more susceptible to HIV infection than CD4 T cells specific to certain other vectors, such asCytomegalovirus[65]andCanarypox.[66]
By comparison, aSciencearticle reported that China had approvedCanSino'sEbola vaccinebased on an Ad5 vector. It was tested inSierra Leone,which had high HIVprevalence,making it more likely for such problems to be detected. CanSino's CEO said "we haven't seen anything with the Ebola vaccine" and speculated that HIV susceptibility might be limited to Ad5 vaccines which produced HIV proteins. In research reported inThe Lancetin May, the company's researchers acknowledged the possibility, called it "controversial" and said they would watch for it in thecompany's COVID-19 vaccine candidate'strials.[51][55]It is not known to what extentLGBT discrimination in Sierra Leonecould have contributed to masking a possible causal link in the Ebola vaccine trial; while the Step trial enrolled mainly homosexual and bisexual men, the Phambili trial enrolled mainly heterosexual men and women and still found an apparent connection.[citation needed]
See also
[edit]References
[edit]- ^Padilla-Sanchez V (2021-07-24),Adenovirus D26 Structural Model at Atomic Resolution,doi:10.5281/zenodo.5132873,retrieved2021-07-24
- ^abcdefghijklm"9.11H: Double-Stranded DNA Viruses- Adenoviruses".Biology LibreTexts.25 June 2017.Retrieved6 January2021.
- ^Rowe WP,Huebner RJ,Gilmore LK, Parrott RH, Ward TG (December 1953). "Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture".Proceedings of the Society for Experimental Biology and Medicine.84(3): 570–3.doi:10.3181/00379727-84-20714.PMID13134217.S2CID3097955.
- ^"Virus Taxonomy: 2020 Release".International Committee on Taxonomy of Viruses (ICTV). March 2021.Retrieved22 May2021.
- ^Dhingra A, Hage E, Ganzenmueller T, Böttcher S, Hofmann J, Hamprecht K, et al. (January 2019)."Molecular Evolution of Human Adenovirus (HAdV) Species C".Scientific Reports.9(1): 1039.Bibcode:2019NatSR...9.1039D.doi:10.1038/s41598-018-37249-4.PMC6355881.PMID30705303.
- ^Walsh MP, Seto J, Liu EB, Dehghan S, Hudson NR, Lukashev AN, et al. (October 2011)."Computational analysis of two species C human adenoviruses provides evidence of a novel virus".Journal of Clinical Microbiology.49(10): 3482–90.doi:10.1128/JCM.00156-11.PMC3187342.PMID21849694.
- ^Robinson CM, Singh G, Henquell C, Walsh MP, Peigue-Lafeuille H, Seto D, et al. (January 2011)."Computational analysis and identification of an emergent human adenovirus pathogen implicated in a respiratory fatality".Virology.409(2): 141–7.doi:10.1016/j.virol.2010.10.020.PMC3006489.PMID21056888.
- ^Singh G, Robinson CM, Dehghan S, Schmidt T, Seto D, Jones MS, et al. (April 2012)."Overreliance on the hexon gene, leading to misclassification of human adenoviruses".Journal of Virology.86(8): 4693–5.doi:10.1128/jvi.06969-11.PMC3318657.PMID22301156.
- ^Singh G, Zhou X, Lee JY, Yousuf MA, Ramke M, Ismail AM, et al. (November 2015)."Recombination of the epsilon determinant and corneal tropism: Human adenovirus species D types 15, 29, 56, and 69".Virology.485:452–9.doi:10.1016/j.virol.2015.08.018.PMC4619159.PMID26343864.
- ^Lee B, Damon CF, Platts-Mills JA (October 2020)."Pediatric acute gastroenteritis associated with adenovirus 40/41 in low-income and middle-income countries".Current Opinion in Infectious Diseases.33(5): 398–403.doi:10.1097/QCO.0000000000000663.PMC8286627.PMID32773498.
- ^Jones MS, Harrach B, Ganac RD, Gozum MM, Dela Cruz WP, Riedel B, et al. (June 2007)."New adenovirus species found in a patient presenting with gastroenteritis".Journal of Virology.81(11): 5978–84.doi:10.1128/JVI.02650-06.PMC1900323.PMID17360747.
- ^"Adenovirus | Home | CDC".www.cdc.gov.Centers for Disease control and prevention. 31 January 2020.Retrieved6 January2021.
- ^Voss JD, Atkinson RL, Dhurandhar NV (November 2015)."Role of adenoviruses in obesity".Reviews in Medical Virology.25(6): 379–87.doi:10.1002/rmv.1852.PMID26352001.S2CID5370331.
- ^Harrach B, Benkő M (2021)."Adenoviruses (Adenoviridae)".Encyclopedia of Virology.Vol. 2. Elsevier.ISBN978-0-12-814516-6.Retrieved12 March2021.
- ^Reddy VS, Natchiar SK, Stewart PL, Nemerow GR (August 2010)."Crystal structure of human adenovirus at 3.5 A resolution".Science.329(5995): 1071–5.Bibcode:2010Sci...329.1071R.doi:10.1126/science.1187292.PMC2929978.PMID20798318.
- Lay summary in:"Scientists unveil structure of adenovirus, the largest high-resolution complex ever found".Science Daily.August 28, 2010.
- ^Harrison SC (August 2010). "Virology. Looking inside adenovirus".Science.329(5995): 1026–7.Bibcode:2010Sci...329.1026H.doi:10.1126/science.1194922.PMID20798308.S2CID206528739.
- ^Acheson NH (2011).Fundamentals of molecular virology(2nd ed.). Hoboken, NJ: Wiley.ISBN978-0-470-90059-8.
- ^Wu E, Nemerow GR (April 2004). "Virus yoga: the role of flexibility in virus host cell recognition".Trends in Microbiology.12(4): 162–9.doi:10.1016/j.tim.2004.02.005.PMID15051066.
- ^Flint J, Skalka AM, Rall GF, Racaniello VR (2015).Principles of Virology.Molecular Biology. Vol. I. American Society of Microbiology.doi:10.1128/9781555818951.ISBN978-1-55581-933-0.
- ^Moyer CL, Wiethoff CM, Maier O, Smith JG, Nemerow GR (March 2011)."Functional genetic and biophysical analyses of membrane disruption by human adenovirus".Journal of Virology.85(6): 2631–2641.doi:10.1128/JVI.02321-10.PMC3067937.PMID21209115.
- ^Meier O, Greber UF (February 2004)."Adenovirus endocytosis".The Journal of Gene Medicine.6(Suppl 1): S152–S163.doi:10.1002/jgm.553.PMID14978758.S2CID22241820.
- ^Schwartz U, Komatsu T, Huber C, Lagadec F, Baumgartl C, Silberhorn E, et al. (2023)."Changes in adenoviral chromatin organization precede early gene activation upon infection".The EMBO Journal.42(19): e114162.doi:10.15252/embj.2023114162.PMC10548178.PMID37641864.
- ^abcLee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, et al. (June 2017)."Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine".Genes & Diseases.4(2): 43–63.doi:10.1016/j.gendis.2017.04.001.PMC5609467.PMID28944281.
- ^Singh G, Ismail AM, Lee JY, Ramke M, Lee JS, Dyer DW, et al. (February 2019)."Divergent Evolution of E1A CR3 in Human Adenovirus Species D".Viruses.11(2): 143.doi:10.3390/v11020143.PMC6409611.PMID30744049.
- ^abYamamoto H, Shimojo H (August 1971). "Multiplicity reactivation of human adenovirus type 12 and simian virus 40 irradiated by ultraviolet light".Virology.45(2): 529–31.doi:10.1016/0042-6822(71)90355-2.PMID4328814.
- ^Michod RE, Bernstein H, Nedelcu AM (May 2008). "Adaptive value of sex in microbial pathogens".Infection, Genetics and Evolution.8(3): 267–85.Bibcode:2008InfGE...8..267M.doi:10.1016/j.meegid.2008.01.002.PMID18295550.
- ^Wang CC, Prather KA, Sznitman J, Jimenez JL, Lakdawala SS, Tufekci Z, et al. (August 2021)."Airborne transmission of respiratory viruses".Science.373(6558): eabd9149.doi:10.1126/science.abd9149.PMC8721651.PMID34446582.
- ^Wan YY, Leon RP, Marks R, Cham CM, Schaack J, Gajewski TF, et al. (December 2000)."Transgenic expression of the coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naive T cells".Proceedings of the National Academy of Sciences of the United States of America.97(25): 13784–13789.Bibcode:2000PNAS...9713784W.doi:10.1073/pnas.250356297.PMC17653.PMID11095726.
- ^Chen LH, Wu ZQ, Hu YF, Yang F, Yang J, Jin Q (June 2012). "[Genetic diversity of adenoviruses in bats of China]".Bing du Xue Bao = Chinese Journal of Virology.28(4): 403–8.PMID22978165.
- ^Li Y, Ge X, Zhang H, Zhou P, Zhu Y, Zhang Y, et al. (April 2010)."Host range, prevalence, and genetic diversity of adenoviruses in bats".Journal of Virology.84(8): 3889–97.doi:10.1128/JVI.02497-09.PMC2849498.PMID20089640.
- ^abFenner FJ, Gibbs EP, Murphy FA, Rott R, Studdert MJ, White DO (1993).Veterinary Virology(2nd ed.). Academic Press, Inc.ISBN978-0-12-253056-2.
- ^Goldstein T, Colegrove KM, Hanson M, Gulland FM (May 2011)."Isolation of a novel adenovirus from California sea lions Zalophus californianus".Diseases of Aquatic Organisms.94(3): 243–8.doi:10.3354/dao02321.PMID21790072.
- ^"Inclusion Body Hepatitis and Hepatitis Hydropericardium Syndrome in Poultry - Poultry".Veterinary Manual.
- ^Kumar R, Kumar V, Asthana M, Shukla SK, Chandra R (January 2010)."Isolation and identification of a fowl adenovirus from wild Black Kites (Milvus migrans)".Journal of Wildlife Diseases.46(1): 272–6.doi:10.7589/0090-3558-46.1.272.PMID20090043.
- ^Chen EC, Yagi S, Kelly KR, Mendoza SP, Tarara RP, Canfield DR, et al. (July 2011). Nemerow GR (ed.)."Cross-species transmission of a novel adenovirus associated with a fulminant pneumonia outbreak in a new world monkey colony".PLOS Pathogens.7(7): e1002155.doi:10.1371/journal.ppat.1002155.PMC3136464.PMID21779173.
- ^ab"Adenovirus | Prevention and Treatment | CDC".2019-09-03.
- ^"USAMRMC protects Soldiers against unseen enemy".
- ^"Default - Stanford Children's Health".Archived fromthe originalon April 3, 2020.
- ^"Adenovirus | Clinical Diagnosis | CDC".2019-08-29.
- ^"Croup".The Lecturio Medical Concept Library.30 April 2020.Retrieved11 July2021.
- ^Uhnoo I, Svensson L, Wadell G (September 1990). "Enteric adenoviruses".Baillière's Clinical Gastroenterology.4(3): 627–42.doi:10.1016/0950-3528(90)90053-j.PMID1962727.
- ^Burkholder A (2007-12-19)."A killer cold? Even the healthy may be vulnerable".CNN.Retrieved2007-12-19.
- ^Hosseini SM, Mirhosseini SM, Taghian M, Salehi M, Farahani MM, Bakhtiari F, et al. (October 2018). "First evidence of the presence of adenovirus type 8 in myocardium of patients with severe idiopathic dilated cardiomyopathy".Archives of Virology.163(10): 2895–2897.doi:10.1007/s00705-018-3942-3.PMID30022238.S2CID49870344.
- ^Fox M (January 28, 2018)."Adenovirus looks like flu, acts like flu, but it's not influenza".NBC News.
- ^Thacker EE, Nakayama M, Smith BF, Bird RC, Muminova Z, Strong TV, et al. (November 2009)."A genetically engineered adenovirus vector targeted to CD40 mediates transduction of canine dendritic cells and promotes antigen-specific immune responses in vivo".Vaccine.27(50): 7116–24.doi:10.1016/j.vaccine.2009.09.055.PMC2784276.PMID19786146.
- ^Harrington KJ, Vile RG, Pandha HS, eds. (May 2008).Viral Therapy of Cancer.Hoboken, N.J.: Wiley. pp.1–13.ISBN978-0-470-01922-1.
- ^Xin KQ, Sekimoto Y, Takahashi T, Mizuguchi H, Ichino M, Yoshida A, et al. (May 2007). "Chimeric adenovirus 5/35 vector containing the clade C HIV gag gene induces a cross-reactive immune response against HIV".Vaccine.25(19): 3809–15.doi:10.1016/j.vaccine.2007.01.117.PMID17386962.
- ^Naskalska A, Szolajska E, Chaperot L, Angel J, Plumas J, Chroboczek J (December 2009). "Influenza recombinant vaccine: matrix protein M1 on the platform of the adenovirus dodecahedron".Vaccine.27(52): 7385–93.doi:10.1016/j.vaccine.2009.09.021.PMID19766576.
- ^Nóbrega C (2020).A handbook of gene and cell therapy.Liliana Mendonça, Carlos A. Matos. Cham: Springer.ISBN978-3-030-41333-0.OCLC1163431307.
- ^Cross R (12 May 2020)."Adenoviral vectors are the new COVID-19 vaccine front-runners. Can they overcome their checkered past?".Chemical & Engineering News.98(19).Retrieved15 December2020.
- ^abCohen J (19 October 2020)."Could certain COVID-19 vaccines leave people more vulnerable to the AIDS virus?".American Association for the Advancement of Science. Science (magazine).Retrieved15 December2020.
- ^Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. (August 2020)."Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial".Lancet.396(10249): 467–478.doi:10.1016/S0140-6736(20)31604-4.PMC7445431.PMID32702298.
- ^"The Oxford/AstraZeneca COVID-19 vaccine: what you need to know".www.who.int.Retrieved2021-03-07.
- ^ab"An Open Study of the Safety, Tolerability and Immunogenicity of the Drug" Gam-COVID-Vac "Vaccine Against COVID-19".Clinical Trials.22 June 2020.Retrieved22 December2020.
- ^abZhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, et al. (June 2020)."Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial".Lancet.395(10240): 1845–1854.doi:10.1016/s0140-6736(20)31208-3.PMC7255193.PMID32450106.
- ^"Experimental Nasal Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2".Sci News.27 August 2020.Retrieved28 August2020.
- ^Hassan AO, Kafai NM, Dmitriev IP, Fox JM, Smith BK, Harvey IB, et al. (October 2020)."A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2".Cell.183(1): 169–184.e13.doi:10.1016/j.cell.2020.08.026.PMC7437481.PMID32931734.
- ^Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatulin AI, Shcheblyakov DV, Dzharullaeva AS, et al. (September 2020)."Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia".Lancet.396(10255): 887–897.doi:10.1016/S0140-6736(20)31866-3.PMC7471804.PMID32896291.
- ^Fausther-Bovendo H,Kobinger GP(2014)."Pre-existing immunity against Ad vectors: humoral, cellular, and innate response, what's important?".Human Vaccines & Immunotherapeutics.10(10): 2875–84.doi:10.4161/hv.29594.PMC5443060.PMID25483662.
- ^Collins S (9 December 2020)."HIV risk from some COVID-19 vaccines might be unlikely due to rarity of vector viruses involved".HIV i-BASE.Retrieved15 December2020.
- ^Fauci AS, Marovich MA, Dieffenbach CW, Hunter E, Buchbinder SP (2014-04-04)."Immune Activation with HIV Vaccines: Implications of the Adenovirus Vector Experience".Science.344(6179): 49–51.doi:10.1126/science.1250672.ISSN0036-8075.PMC4414116.PMID24700849.
- ^Rosenberg J (25 October 2020)."Researchers Warn of Heightened Risk of HIV With Certain COVID-19 Vaccines".AJMC.Retrieved15 December2020.
- ^Buchbinder SP, McElrath MJ, Dieffenbach C, Corey L (2020-10-31)."Use of adenovirus type-5 vectored vaccines: a cautionary tale".Lancet.396(10260): e68–e69.doi:10.1016/S0140-6736(20)32156-5.PMC7571904.PMID33091364.
- ^Buhl L (2 December 2020)."Most COVID-19 Vaccines Won't Affect HIV Risk: Here's What the Science Tells Us".
- ^Hu H, Eller MA, Zafar S, Zhou Y, Gu M, Wei Z, et al. (2014-09-16)."Preferential infection of human Ad5-specific CD4 T cells by HIV in Ad5 naturally exposed and recombinant Ad5-HIV vaccinated individuals".Proceedings of the National Academy of Sciences of the United States of America.111(37): 13439–44.Bibcode:2014PNAS..11113439H.doi:10.1073/pnas.1400446111.ISSN0027-8424.PMC4169982.PMID25197078.
- ^Auclair S, Liu F, Niu Q, Hou W, Churchyard G, Morgan C, et al. (2018-02-23)."Distinct susceptibility of HIV vaccine vector-induced CD4 T cells to HIV infection".PLOS Pathogens.14(2): e1006888.doi:10.1371/journal.ppat.1006888.ISSN1553-7374.PMC5841825.PMID29474461.
