Atmosphere of Earth

Theatmosphere of Earthis the planetaryatmosphereofEarth,composed of a layer ofgasmixturethat surrounds the Earth'splanetary surface(bothlandsandoceans), known collectively asair,with variable quantities ofsuspendedaerosolsandparticulates(which createweatherfeatures such ascloudsandhazes), all retained byEarth's gravity.The atmosphere serves as a protective buffer between the Earth's surface andouter space,shields the surface from mostmeteoroidsandultravioletsolar radiation,keeps it warm and reducesdiurnal temperature variation(temperature extremes betweendayandnight) through heat retention (greenhouse effect), redistributes heat and moisture among different regions viaair currents,and provides thechemicalandclimateconditions allowinglifeto exist andevolveon Earth.
Bymole fraction(i.e., by quantity ofmolecules), dry air contains 78.08%nitrogen,20.95%oxygen,0.93%argon,0.03%carbon dioxide,and small amounts of othertrace gases.[2]Air also contains a variable amount ofwater vapor,on average around 1% at sea level, and 0.4% over the entire atmosphere. Air composition, temperature andatmospheric pressurevary withaltitude.Within the atmosphere, air suitable for use inphotosynthesisbyterrestrial plantsandrespirationofterrestrial animalsis found only within 12 kilometres (7.5 mi) from the ground.[3]
Earth's early atmosphere consisted ofaccretedgases from thesolar nebula,but the atmosphere changed significantly over time, affected by many factors such asvolcanism,impact events,weatheringand theevolution of life(particularly thephotoautotrophs). Recently,humanactivity has alsocontributed to atmospheric changes,such asclimate change(mainly throughdeforestationandfossil fuel-relatedglobal warming),ozone depletionandacid deposition.
The atmosphere has a mass of about 5.15×1018kg,[4]three quarters of which is within about 11 km (6.8 mi; 36,000 ft) of the surface. The atmosphere becomes thinner with increasing altitude, with no definite boundary between the atmosphere andouter space.TheKármán line,at 100 km (62 mi) or 1.57% of Earth's radius, is often used as the border between the atmosphere and outer space. Atmospheric effects become noticeable duringatmospheric reentryof spacecraft at an altitude of around 120 km (75 mi). Severallayerscan be distinguished in the atmosphere based on characteristics such as temperature and composition, namely thetroposphere,stratosphere,mesosphere,thermosphere(formally theionosphere) andexosphere.
The study of Earth's atmosphere and its processes is calledatmospheric science(aerology), and includes multiple subfields, such asclimatologyandatmospheric physics.Early pioneers in the field includeLéon Teisserenc de BortandRichard Assmann.[5]The study of historic atmosphere is calledpaleoclimatology.
Composition

The three major constituents of Earth's atmosphere arenitrogen,oxygen,andargon.Water vapor accounts for roughly 0.25% of the atmosphere by mass. The concentration of water vapor (a greenhouse gas) varies significantly from around 10 ppm by mole fraction in the coldest portions of the atmosphere to as much as 5% by mole fraction in hot, humid air masses, and concentrations of other atmospheric gases are typically quoted in terms of dry air (without water vapor).[9]: 8 The remaining gases are often referred to as trace gases,[10]among which are othergreenhouse gases,principally carbon dioxide, methane, nitrous oxide, and ozone. Besides argon, already mentioned, othernoble gases,neon,helium,krypton,andxenonare also present. Filtered air includes trace amounts of many otherchemical compounds.Many substances of natural origin may be present in locally and seasonally variable small amounts asaerosolsin an unfiltered air sample, includingdustof mineral and organic composition,pollenandspores,sea spray,andvolcanic ash.Various industrialpollutantsalso may be present as gases or aerosols, such aschlorine(elemental or in compounds),fluorinecompounds and elementalmercuryvapor. Sulfur compounds such ashydrogen sulfideandsulfur dioxide(SO2) may be derived from natural sources or from industrial air pollution.
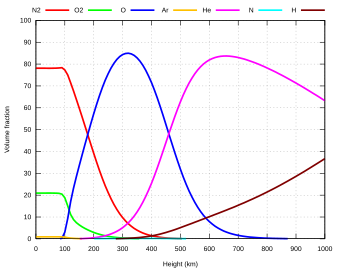
| Dry air | |||
|---|---|---|---|
| Gas | Mole fraction(A) | ||
| Name | Formula | inppm(B) | in% |
| Nitrogen | N2 | 780,840 | 78.084 |
| Oxygen | O2 | 209,460 | 20.946 |
| Argon | Ar | 9,340 | 0.9340 |
| Carbon dioxide (April 2022)(C)[14] |
CO2 | 417 | 0.0417 |
| Neon | Ne | 18.18 | 0.001818 |
| Helium | He | 5.24 | 0.000524 |
| Methane (2022)(C)[15] |
CH4 | 1.91 | 0.000191 |
| Krypton | Kr | 1.14 | 0.000114 |
| If air is not dry: | |||
| Water vapor(D) | H2O | 0–30,000(D) | 0–3%(E) |
notes:
| |||
The averagemolecular weightof dry air, which can be used to calculate densities or to convert between mole fraction and mass fraction, is about 28.946[16]or 28.96[17][18]g/mol. This is decreased when the air is humid.
The relative concentration of gases remains constant until about 10,000 m (33,000 ft).[19]
Stratification

In general, air pressure and density decrease with altitude in the atmosphere. However, the temperature has a more complicated profile with altitude, and may remain relatively constant or even increase with altitude in some regions (see thetemperaturesection). Because the general pattern of the temperature/altitude profile, orlapse rate,is constant and measurable by means of instrumentedballoon soundings,the temperature behavior provides a useful metric to distinguish atmospheric layers. In this way, Earth's atmosphere can be divided (called atmospheric stratification) into five main layers: troposphere, stratosphere, mesosphere, thermosphere, and exosphere.[20]The altitudes of the five layers are:
- Exosphere: 700 to 10,000 km (440 to 6,200 miles)[21]
- Thermosphere: 80 to 700 km (50 to 440 miles)[22]
- Mesosphere: 50 to 80 km (31 to 50 miles)
- Stratosphere: 12 to 50 km (7 to 31 miles)
- Troposphere: 0 to 12 km (0 to 7 miles)[23]
Exosphere
The exosphere is the outermost layer of Earth's atmosphere (though it is so tenuous that some scientists consider it to be part of interplanetary space rather than part of the atmosphere). It extends from thethermopause(also known as the "exobase" ) at the top of thethermosphereto a poorly defined boundary with thesolar windandinterplanetary medium.The altitude of the exobase varies from about 500 kilometres (310 mi; 1,600,000 ft) to about 1,000 kilometres (620 mi) in times of higher incoming solar radiation.[24]
The upper limit varies depending on the definition. Various authorities consider it to end at about 10,000 kilometres (6,200 mi)[25]or about 190,000 kilometres (120,000 mi)—about halfway to the moon, where the influence of Earth's gravity is about the same asradiation pressurefrom sunlight.[24]Thegeocoronavisible in the far ultraviolet (caused by neutral hydrogen) extends to at least 100,000 kilometres (62,000 mi).[24]
This layer is mainly composed of extremely low densities of hydrogen, helium and several heavier molecules including nitrogen, oxygen and carbon dioxide closer to the exobase. The atoms and molecules are so far apart that they can travel hundreds of kilometres without colliding with one another. Thus, the exosphere no longer behaves like a gas, and the particles constantlyescape into space.These free-moving particles followballistictrajectoriesand may migrate in and out of themagnetosphereor the solar wind. Every second, the Earth loses about 3 kg of hydrogen, 50 g of helium, and much smaller amounts of other constituents.[26]
The exosphere is too far above Earth formeteorologicalphenomena to be possible. However, Earth'sauroras—the aurora borealis (northern lights) and aurora australis (southern lights)—sometimes occur in the lower part of the exosphere, where they overlap into the thermosphere. The exosphere contains many of theartificial satellitesthatorbitEarth.
Thermosphere
The thermosphere is the second-highest layer of Earth's atmosphere. It extends from the mesopause (which separates it from the mesosphere) at an altitude of about 80 km (50 mi; 260,000 ft) up to thethermopauseat an altitude range of 500–1000 km (310–620 mi; 1,600,000–3,300,000 ft). The height of the thermopause varies considerably due to changes in solar activity.[22]Because the thermopause lies at the lower boundary of the exosphere, it is also referred to as theexobase.The lower part of the thermosphere, from 80 to 550 kilometres (50 to 342 mi) above Earth's surface, contains theionosphere.
The temperature of the thermosphere gradually increases with height and can rise as high as 1500 °C (2700 °F), though the gas molecules are so far apart that itstemperature in the usual senseis not very meaningful. The air is so rarefied that an individual molecule (ofoxygen,for example) travels an average of 1 kilometre (0.62 mi; 3300 ft) between collisions with other molecules.[27]Although the thermosphere has a high proportion of molecules with high energy, it would not feel hot to a human in direct contact, because its density is too low to conduct a significant amount of energy to or from the skin.
This layer is completely cloudless and free of water vapor. However, non-hydrometeorological phenomena such as theaurora borealisandaurora australisare occasionally seen in the thermosphere. TheInternational Space Stationorbits in this layer, between 350 and 420 km (220 and 260 mi). It is this layer where many of the satellites orbiting the Earth are present.
Mesosphere

The mesosphere is the third highest layer of Earth's atmosphere, occupying the region above the stratosphere and below the thermosphere. It extends from the stratopause at an altitude of about 50 km (31 mi; 160,000 ft) to the mesopause at 80–85 km (50–53 mi; 260,000–280,000 ft) above sea level.
Temperatures drop with increasing altitude to themesopausethat marks the top of this middle layer of the atmosphere. It is the coldest place on Earth and has an average temperature around −85°C(−120°F;190K).[28][29]
Just below the mesopause, the air is so cold that even the very scarce water vapor at this altitude can condense into polar-mesosphericnoctilucent cloudsof ice particles. These are the highest clouds in the atmosphere and may be visible to the naked eye if sunlight reflects off them about an hour or two after sunset or similarly before sunrise. They are most readily visible when the Sun is around 4 to 16 degrees below the horizon. Lightning-induced discharges known astransient luminous events(TLEs) occasionally form in the mesosphere above troposphericthunderclouds.The mesosphere is also the layer where mostmeteorsburn up upon atmospheric entrance. It is too high above Earth to be accessible to jet-powered aircraft and balloons, and too low to permit orbital spacecraft. The mesosphere is mainly accessed bysounding rocketsandrocket-powered aircraft.
Stratosphere
The stratosphere is the second-lowest layer of Earth's atmosphere. It lies above the troposphere and is separated from it by thetropopause.This layer extends from the top of the troposphere at roughly 12 km (7.5 mi; 39,000 ft) above Earth's surface to thestratopauseat an altitude of about 50 to 55 km (31 to 34 mi; 164,000 to 180,000 ft).
The atmospheric pressure at the top of the stratosphere is roughly 1/1000 thepressure at sea level.It contains theozone layer,which is the part of Earth's atmosphere that contains relatively high concentrations of that gas. The stratosphere defines a layer in which temperatures rise with increasing altitude. This rise in temperature is caused by the absorption ofultraviolet radiation(UV) from the Sun by the ozone layer, which restricts turbulence and mixing. Although the temperature may be −60 °C (−76 °F; 210 K) at the tropopause, the top of the stratosphere is much warmer, and may be near 0 °C.[30]
The stratospheric temperature profile creates very stable atmospheric conditions, so the stratosphere lacks the weather-producing air turbulence that is so prevalent in the troposphere. Consequently, the stratosphere is almost completely free of clouds and other forms of weather. However, polar stratospheric ornacreous cloudsare occasionally seen in the lower part of this layer of the atmosphere where the air is coldest. The stratosphere is the highest layer that can be accessed byjet-powered aircraft.
Troposphere

The troposphere is the lowest layer of Earth's atmosphere. It extends from Earth's surface to an average height of about 12 km (7.5 mi; 39,000 ft), although thisaltitudevaries from about 9 km (5.6 mi; 30,000 ft) at thegeographic polesto 17 km (11 mi; 56,000 ft) at theEquator,[23]with some variation due to weather. The troposphere is bounded above by thetropopause,a boundary marked in most places by atemperature inversion(i.e. a layer of relatively warm air above a colder one), and in others by a zone that isisothermalwith height.[31][32]
Although variations do occur, the temperature usually declines with increasing altitude in the troposphere because the troposphere is mostly heated through energy transfer from the surface. Thus, the lowest part of the troposphere (i.e. Earth's surface) is typically the warmest section of the troposphere. This promotes vertical mixing (hence, the origin of its name in the Greek word τρόπος,tropos,meaning "turn" ). The troposphere contains roughly 80% of themassof Earth's atmosphere.[33]The troposphere is denser than all its overlying layers because a larger atmospheric weight sits on top of the troposphere and causes it to be most severely compressed. Fifty percent of the total mass of the atmosphere is located in the lower 5.6 km (3.5 mi; 18,000 ft) of the troposphere.
Nearly all atmospheric water vapor or moisture is found in the troposphere, so it is the layer where most of Earth's weather takes place. It has basically all the weather-associated cloud genus types generated by active wind circulation, although very tall cumulonimbus thunder clouds can penetrate the tropopause from below and rise into the lower part of the stratosphere. Most conventionalaviationactivity takes place in the troposphere, and it is the only layer accessible bypropeller-driven aircraft.
Other layers
Within the five principal layers above, which are largely determined by temperature, several secondary layers may be distinguished by other properties:
- Theozone layeris contained within the stratosphere. In this layerozoneconcentrations are about 2 to 8 parts per million, which is much higher than in the lower atmosphere but still very small compared to the main components of the atmosphere. It is mainly located in the lower portion of the stratosphere from about 15–35 km (9.3–21.7 mi; 49,000–115,000 ft), though the thickness varies seasonally and geographically. About 90% of the ozone in Earth's atmosphere is contained in the stratosphere.
- Theionosphereis a region of the atmosphere that is ionized by solar radiation. It is responsible forauroras.During daytime hours, it stretches from 50 to 1,000 km (31 to 621 mi; 160,000 to 3,280,000 ft) and includes the mesosphere, thermosphere, and parts of the exosphere. However, ionization in the mesosphere largely ceases during the night, so auroras are normally seen only in the thermosphere and lower exosphere. The ionosphere forms the inner edge of themagnetosphere.It has practical importance because it influences, for example, radio propagation on Earth.
- Thehomosphereandheterosphereare defined by whether the atmospheric gases are well mixed. The surface-based homosphere includes the troposphere, stratosphere, mesosphere, and the lowest part of the thermosphere, where the chemical composition of the atmosphere does not depend on molecular weight because the gases are mixed by turbulence.[34]This relatively homogeneous layer ends at theturbopausefound at about 100 km (62 mi; 330,000 ft), the veryedge of spaceitself as accepted by theFAI,which places it about 20 km (12 mi; 66,000 ft) above the mesopause.
- Above this altitude lies the heterosphere, which includes the exosphere and most of the thermosphere. Here, the chemical composition varies with altitude. This is because thedistance that particles can move without colliding with one anotheris large compared with the size of motions that cause mixing. This allows the gases to stratify by molecular weight, with the heavier ones, such as oxygen and nitrogen, present only near the bottom of the heterosphere. The upper part of the heterosphere is composed almost completely of hydrogen, the lightest element.[35]
- Theplanetary boundary layeris the part of the troposphere that is closest to Earth's surface and is directly affected by it, mainly throughturbulent diffusion.During the day the planetary boundary layer usually is well-mixed, whereas at night it becomes stably stratified with weak or intermittent mixing. The depth of the planetary boundary layer ranges from as little as about 100 metres (330 ft) on clear, calm nights to 3,000 m (9,800 ft) or more during the afternoon in dry regions.
The average temperature of the atmosphere at Earth's surface is 14 °C (57 °F; 287 K)[36]or 15 °C (59 °F; 288 K),[37]depending on the reference.[38][39][40]
Physical properties
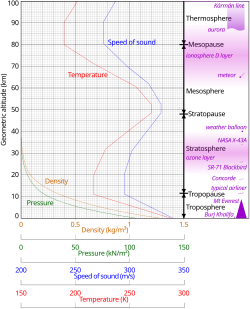
Pressure and thickness
The average atmospheric pressure at sea level is defined by theInternational Standard Atmosphereas 101325pascals(760.00Torr;14.6959psi;760.00mmHg). This is sometimes referred to as a unit ofstandard atmospheres (atm).Total atmospheric mass is 5.1480×1018kg (1.135×1019lb),[42]about 2.5% less than would be inferred from the average sea level pressure and Earth's area of 51007.2 megahectares, this portion being displaced by Earth's mountainous terrain. Atmospheric pressure is the total weight of the air above unit area at the point where the pressure is measured. Thus air pressure varies with location andweather.
If the entire mass of the atmosphere had a uniform density equal to sea level density (about 1.2 kg per m3) from sea level upwards, it would terminate abruptly at an altitude of 8.50 km (27,900 ft).
Air pressure actually decreases exponentially with altitude, dropping by half every 5.6 km (18,000 ft) or by a factor of 1/e(0.368) every 7.64 km (25,100 ft), (this is called thescale height) -- for altitudes out to around 70 km (43 mi; 230,000 ft). However, the atmosphere is more accurately modeled with a customized equation for each layer that takes gradients of temperature, molecular composition, solar radiation and gravity into account. At heights over 100 km, an atmosphere may no longer be well mixed. Then each chemical species has its own scale height.
In summary, the mass of Earth's atmosphere is distributed approximately as follows:[43]
- 50% is below 5.6 km (18,000 ft).
- 90% is below 16 km (52,000 ft).
- 99.99997% is below 100 km (62 mi; 330,000 ft), theKármán line.By international convention, this marks the beginning of space where human travelers are consideredastronauts.
By comparison, the summit ofMount Everestis at 8,848 m (29,029 ft); commercialairlinerstypically cruise between 10 and 13 km (33,000 and 43,000 ft) where the lower density and temperature of the air improve fuel economy;weather balloonsreach 30.4 km (100,000 ft) and above; and the highestX-15flight in 1963 reached 108.0 km (354,300 ft).
Even above the Kármán line, significant atmospheric effects such asaurorasstill occur.Meteorsbegin to glow in this region, though the larger ones may not burn up until they penetrate more deeply. The various layers of Earth'sionosphere,important toHF radiopropagation, begin below 100 km and extend beyond 500 km. By comparison, theInternational Space StationandSpace Shuttletypically orbit at 350–400 km, within theF-layerof the ionosphere where they encounter enoughatmospheric dragto require reboosts every few months, otherwise,orbital decaywill occur resulting in a return to Earth. Depending on solar activity, satellites can experience noticeable atmospheric drag at altitudes as high as 700–800 km.
Temperature
The division of the atmosphere into layers mostly by reference to temperature is discussed above. Temperature decreases with altitude starting at sea level, but variations in this trend begin above 11 km, where the temperature stabilizes over a large vertical distance through the rest of the troposphere. In thestratosphere,starting above about 20 km, the temperature increases with height, due to heating within the ozone layer caused by the capture of significantultravioletradiation from theSunby the dioxygen and ozone gas in this region. Still another region of increasing temperature with altitude occurs at very high altitudes, in the aptly-namedthermosphereabove 90 km.
Speed of sound
Because in anideal gasof constant composition thespeed of sounddepends only on temperature and not on pressure or density, the speed of sound in the atmosphere with altitude takes on the form of the complicated temperature profile (see illustration to the right), and does not mirror altitudinal changes in density or pressure.
Density and mass

The density of air at sea level is about 1.2 kg/m3(1.2 g/L, 0.0012 g/cm3). Density is not measured directly but is calculated from measurements of temperature, pressure and humidity using the equation of state for air (a form of theideal gas law). Atmospheric density decreases as the altitude increases. This variation can be approximately modeled using thebarometric formula.More sophisticated models are used to predict the orbital decay of satellites.
The average mass of the atmosphere is about 5 quadrillion (5×1015)tonnesor 1/1,200,000 the mass of Earth. According to the AmericanNational Center for Atmospheric Research,"The total mean mass of the atmosphere is 5.1480×1018kg with an annual range due to water vapor of 1.2 or 1.5×1015kg, depending on whether surface pressure or water vapor data are used; somewhat smaller than the previous estimate. The mean mass of water vapor is estimated as 1.27×1016kg and the dry air mass as 5.1352 ±0.0003×1018kg. "
Tabulated properties
Table of physical and thermal properties of air at atmospheric pressure:[45][46]
| Temperature [K] | Density [kg/m3] | Specific heat [J/(kg⋅°C)] | Dynamic viscosity [kg/(m⋅s)] | Kinematic viscosity [m2/s] | Thermal conductivity [W/(m⋅°C)] | Thermal diffusivity [m2/s] | Prandtl number [1] | Bulk modulus [K−1] |
|---|---|---|---|---|---|---|---|---|
| 100 | 3.601 | 1026.6 | 6.92×10−6 | 1.92×10−6 | 0.000925 | 2.50×10−6 | 0.77 | 0.01 |
| 150 | 2.3675 | 1009.9 | 1.03×10−5 | 4.34×10−6 | 0.013735 | 5.75×10−6 | 0.753 | 0.006667 |
| 200 | 1.7684 | 1006.1 | 1.33×10−5 | 7.49×10−6 | 0.01809 | 1.02×10−5 | 0.738 | 0.005 |
| 250 | 1.4128 | 1005.3 | 1.60×10−5 | 1.13×10−5 | 0.02227 | 1.57×10−5 | 0.722 | 0.004 |
| 300 | 1.1774 | 1005.7 | 1.85×10−5 | 1.57×10−5 | 0.02624 | 2.22×10−5 | 0.708 | 0.003333 |
| 350 | 0.998 | 1009 | 2.08×10−5 | 2.08×10−5 | 0.03003 | 2.98×10−5 | 0.697 | 0.002857 |
| 400 | 0.8826 | 1014 | 2.29×10−5 | 2.59×10−5 | 0.03365 | 3.76×10−5 | 0.689 | 0.0025 |
| 450 | 0.7833 | 1020.7 | 2.48×10−5 | 3.17×10−5 | 0.03707 | 4.22×10−5 | 0.683 | 0.002222 |
| 500 | 0.7048 | 1029.5 | 2.67×10−5 | 3.79×10−5 | 0.04038 | 5.56×10−5 | 0.68 | 0.002 |
| 550 | 0.6423 | 1039.2 | 2.85×10−5 | 4.43×10−5 | 0.0436 | 6.53×10−5 | 0.68 | 0.001818 |
| 600 | 0.5879 | 1055.1 | 3.02×10−5 | 5.13×10−5 | 0.04659 | 7.51×10−5 | 0.68 | 0.001667 |
| 650 | 0.543 | 1063.5 | 3.18×10−5 | 5.85×10−5 | 0.04953 | 8.58×10−5 | 0.682 | 0.001538 |
| 700 | 0.503 | 1075.2 | 3.33×10−5 | 6.63×10−5 | 0.0523 | 9.67×10−5 | 0.684 | 0.001429 |
| 750 | 0.4709 | 1085.6 | 3.48×10−5 | 7.39×10−5 | 0.05509 | 1.08×10−4 | 0.686 | 0.001333 |
| 800 | 0.4405 | 1097.8 | 3.63×10−5 | 8.23×10−5 | 0.05779 | 1.20×10−4 | 0.689 | 0.00125 |
| 850 | 0.4149 | 1109.5 | 3.77×10−5 | 9.08×10−5 | 0.06028 | 1.31×10−4 | 0.692 | 0.001176 |
| 900 | 0.3925 | 1121.2 | 3.90×10−5 | 9.93×10−5 | 0.06279 | 1.43×10−4 | 0.696 | 0.001111 |
| 950 | 0.3716 | 1132.1 | 4.02×10−5 | 1.08×10−4 | 0.06525 | 1.55×10−4 | 0.699 | 0.001053 |
| 1000 | 0.3524 | 1141.7 | 4.15×10−5 | 1.18×10−4 | 0.06753 | 1.68×10−4 | 0.702 | 0.001 |
| 1100 | 0.3204 | 1160 | 4.44×10−5 | 1.39×10−4 | 0.0732 | 1.97×10−4 | 0.704 | 0.000909 |
| 1200 | 0.2947 | 1179 | 4.69×10−5 | 1.59×10−4 | 0.0782 | 2.25×10−4 | 0.707 | 0.000833 |
| 1300 | 0.2707 | 1197 | 4.93×10−5 | 1.82×10−4 | 0.0837 | 2.58×10−4 | 0.705 | 0.000769 |
| 1400 | 0.2515 | 1214 | 5.17×10−5 | 2.06×10−4 | 0.0891 | 2.92×10−4 | 0.705 | 0.000714 |
| 1500 | 0.2355 | 1230 | 5.40×10−5 | 2.29×10−4 | 0.0946 | 3.26×10−4 | 0.705 | 0.000667 |
| 1600 | 0.2211 | 1248 | 5.63×10−5 | 2.55×10−4 | 0.1 | 3.61×10−4 | 0.705 | 0.000625 |
| 1700 | 0.2082 | 1267 | 5.85×10−5 | 2.81×10−4 | 0.105 | 3.98×10−4 | 0.705 | 0.000588 |
| 1800 | 0.197 | 1287 | 6.07×10−5 | 3.08×10−4 | 0.111 | 4.38×10−4 | 0.704 | 0.000556 |
| 1900 | 0.1858 | 1309 | 6.29×10−5 | 3.39×10−4 | 0.117 | 4.81×10−4 | 0.704 | 0.000526 |
| 2000 | 0.1762 | 1338 | 6.50×10−5 | 3.69×10−4 | 0.124 | 5.26×10−4 | 0.702 | 0.0005 |
| 2100 | 0.1682 | 1372 | 6.72×10−5 | 4.00×10−4 | 0.131 | 5.72×10−4 | 0.7 | 0.000476 |
| 2200 | 0.1602 | 1419 | 6.93×10−5 | 4.33×10−4 | 0.139 | 6.12×10−4 | 0.707 | 0.000455 |
| 2300 | 0.1538 | 1482 | 7.14×10−5 | 4.64×10−4 | 0.149 | 6.54×10−4 | 0.71 | 0.000435 |
| 2400 | 0.1458 | 1574 | 7.35×10−5 | 5.04×10−4 | 0.161 | 7.02×10−4 | 0.718 | 0.000417 |
| 2500 | 0.1394 | 1688 | 7.57×10−5 | 5.44×10−4 | 0.175 | 7.44×10−4 | 0.73 | 0.0004 |
| 3000 | 0.1135 | 2.726[why?] | 9.55×10−5 | 8.41×10−4 | 0.486 | 1.57×10−3 | 0.536 | 0.0003333 |
Optical properties
Solarradiation(or sunlight) is the energy Earth receives from theSun.Earth also emits radiation back into space, but at longer wavelengths that humans cannot see. Part of the incoming and emitted radiation is absorbed or reflected by the atmosphere.[47][48]In May 2017, glints of light, seen as twinkling from an orbiting satellite a million miles away, were found to bereflected lightfromice crystalsin the atmosphere.[49][50]
Scattering
When light passes through Earth's atmosphere,photonsinteract with it throughscattering.If the light does not interact with the atmosphere, it is calleddirect radiationand is what you see if you were to look directly at the Sun.Indirect radiationis light that has been scattered in the atmosphere. For example, on anovercastday when you cannot see your shadow, there is no direct radiation reaching you, it has all been scattered. As another example, due to a phenomenon calledRayleigh scattering,shorter (blue) wavelengths scatter more easily than longer (red) wavelengths. This is why the sky looks blue; you are seeing scattered blue light. This is also why sunsets are red. Because the Sun is close to the horizon, the Sun's rays pass through more atmosphere than normal before reaching your eye. Much of the blue light has been scattered out, leaving the red light in a sunset.
Absorption

Different molecules absorb different wavelengths of radiation. For example, O2and O3absorb almost all radiation with wavelengths shorter than 300nanometres.Water (H2O) absorbs at many wavelengths above 700 nm. When a molecule absorbs a photon, it increases the energy of the molecule. This heats the atmosphere, but the atmosphere also cools by emitting radiation, as discussed below.
The combinedabsorption spectraof the gases in the atmosphere leave "windows" of lowopacity,allowing the transmission of only certain bands of light. Theoptical windowruns from around 300 nm (ultraviolet-C) up into the range humans can see, thevisible spectrum(commonly called light), at roughly 400–700 nm and continues to theinfraredto around 1100 nm. There are alsoinfraredandradio windowsthat transmit some infrared andradio wavesat longer wavelengths. For example, the radio window runs from about one centimetre to about eleven-metre waves.
Emission
Emissionis the opposite of absorption, it is when an object emits radiation. Objects tend to emit amounts and wavelengths of radiation depending on their "black body"emission curves, therefore hotter objects tend to emit more radiation, with shorter wavelengths. Colder objects emit less radiation, with longer wavelengths. For example, the Sun is approximately 6,000K(5,730°C;10,340°F), its radiation peaks near 500 nm, and is visible to the human eye. Earth is approximately 290 K (17 °C; 62 °F), so its radiation peaks near 10,000 nm, and is much too long to be visible to humans.
Because of its temperature, the atmosphere emits infrared radiation. For example, on clear nights Earth's surface cools down faster than on cloudy nights. This is because clouds (H2O) are strong absorbers and emitters of infrared radiation. This is also why it becomes colder at night at higher elevations.
Thegreenhouse effectis directly related to this absorption and emission effect. Some gases in the atmosphere absorb and emit infrared radiation, but do not interact with sunlight in the visible spectrum. Common examples of these are CO2and H2O.
Refractive index

Therefractive indexof air is close to, but just greater than, 1. Systematic variations in the refractive index can lead to the bending of light rays over long optical paths. One example is that, under some circumstances, observers on board ships can see other vessels just over thehorizonbecause light is refracted in the same direction as thecurvatureof Earth's surface.
The refractive index of air depends on temperature,[51]giving rise to refraction effects when the temperature gradient is large. An example of such effects is themirage.
Circulation
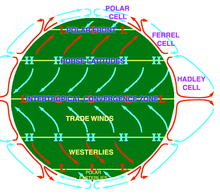
Atmospheric circulationis the large-scale movement of air through the troposphere, and the means (withocean circulation) by which heat is distributed around Earth. The large-scale structure of the atmospheric circulation varies from year to year, but the basic structure remains fairly constant because it is determined by Earth's rotation rate and the difference in solar radiation between the equator and poles.
Evolution of Earth's atmosphere
Earliest atmosphere
The first atmosphere, during theEarly Earth'sHadeaneon,consisted of gases in thesolar nebula,primarilyhydrogen.There were probably simplehydridessuch as those now found in thegas giants(JupiterandSaturn), notablywater vapor,methaneandammonia. During this earliest era numerous collisions with large meteorites heated the atmosphere, driving off the most volatile gases. The largest of these collisions formed the Moon. Large portions of the planet were melted by this impact and the cooling rock outgassed significant amounts of steam which eventually condensed to contribute to ocean water.[52]: 10
Second atmosphere
ThesolidificationofEarth's crustat the end of the Hadean closed off most of theadvectiveheat transferto the surface, causing the atmosphere to cool, whichcondensedmost of the water vapor out of the airprecipitatinginto asuperocean.Furtheroutgassingfromvolcanism,supplemented by gases introduced by hugeasteroidsduring theLate Heavy Bombardment,produced the next Archean atmosphere, consisting largely ofnitrogenpluscarbon dioxideand inert gases.[52]A major part of carbon-dioxide emissions dissolved in water and reacted with metals such as calcium and magnesium during weathering of crustal rocks to form carbonates that were deposited as sediments. Water-related sediments have been found that date from as early as 3.8 billion years ago.[53]
About 3.4 billion years ago, nitrogen formed the major part of the then stable "second atmosphere". The influence of life has to be taken into account rather soon in the history of the atmosphere because hints of early life-forms appear as early as 3.5 billion years ago.[54]How Earth at that time maintained a climate warm enough for liquid water and life, if the early Sun put out 30% lower solar radiance than today, is a puzzle known as the "faint young Sun paradox".
The geological record however shows a continuous relatively warm surface during the complete earlytemperature recordof Earth – with the exception of one cold glacial phase about 2.4 billion years ago. In the lateArcheaneon, an oxygen-containing atmosphere began to develop, apparently produced bycyanobacterialphotosynthesis(seeGreat Oxygenation Event), which have been found asstromatolitefossils from 2.7 billion years ago. The early basic carbon isotopy (isotope ratioproportions) strongly suggests conditions similar to the current, and that the fundamental features of thecarbon cyclebecame established as early as 4 billion years ago.
Ancient sedimentsin theGabondating from between about 2.15 and 2.08 billion years ago provide a record of Earth's dynamic oxygenation evolution. These fluctuations in oxygenation were likely driven by the Lomagundi carbon isotope excursion.[55]
Third atmosphere
The constant re-arrangement ofcontinentsbyplate tectonicsinfluences the long-term evolution of the atmosphere by transferring carbon dioxide to and from large continentalcarbonatestores. Free oxygen did not exist in the atmosphere until about 2.4 billion years ago during theGreat Oxygenation Eventand its appearance is indicated by the end ofbanded iron formations(which signals the depletion ofsubstratesthat can react with oxygen to produceferricdeposits) during the earlyProterozoiceon.
Before this time, any oxygen produced by cyanobacterial photosynthesis was readily removed by theoxidationofreducing substanceson the Earth's surface, notablyferrous iron,sulfurandatmospheric methane.Free oxygen molecules did not start to accumulate in the atmosphere until the rate of production of oxygen began to exceed the availability of reductant materials that removed oxygen. This point signifies a shift from areducing atmosphereto anoxidizingatmosphere. O2showed major variations until reaching a steady state of more than 15% by the end of thePrecambrian,[58]followed by anevolutionary radiationevent known as theAvalon explosion,where complexmetazoanlife forms (including the earliestcnidarians,placozoansandbilaterians) first proliferated. The following time span from 539 million years ago to the present day is thePhanerozoiceon, during the earliestperiodof which, theCambrian,moreactively movingmetazoan life began to appear and rapidly diversify in another radiation event called theCambrian explosion,whoselocomotivemetabolismwas fuelled by the rising oxygen level.
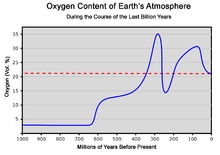
The amount of oxygen in the atmosphere has fluctuated over the last 600 million years, reaching a peak of about 30% around 280 million years ago during theCarboniferousperiod, significantly higher than today's 21%. Two main processes govern changes in the atmosphere: theevolution ofplantsand their increasing role incarbon fixation,and the consumption of oxygen by plants forphotorespirationand their own metabolic needs at night. Breakdown ofpyriteandvolcanic eruptionsrelease sulfur into the atmosphere, which reacts and hence reduces oxygen in the atmosphere. However, volcanic eruptions also release carbon dioxide, which plants can use for oxygenic photosynthesis. The cause of the variation of the amount of oxygen in the atmosphere is not precisely known. Periods with more oxygen in the atmosphere were often associated with more rapid development of animals.
Air pollution
Air pollutionis the introduction of airbornechemicals,particulate matterorbiological materialsthat cause harm or discomfort to organisms.[59]Thepopulation growth,industrializationandmotorizationofhumansocietieshave significantly increased the amount of airbornepollutantsin the Earth's atmosphere, causing noticeable problems such assmogs,acid rainsandpollution-related diseases.Thedepletionofstratosphericozone layer,which shields the surface from harmfulionizingultravioletradiations, is also caused by air pollution, chiefly fromchlorofluorocarbonsand other ozone-depleting substances.
Since 1750, human activity, especially after theIndustrial Revolution,has increased the concentrations of variousgreenhouse gases,most importantly carbon dioxide, methane andnitrous oxide.Greenhouse gas emissions,coupled withdeforestationanddestructionofwetlandsvialoggingandland developments,have caused an observedrise in global temperatures,with the global average surface temperatures being1.1 °Chigher in the 2011–2020 decade than they were in 1850.[60]It has raised concerns of man-madeclimate change,which can have significantenvironmental impactssuch assea level rise,ocean acidification,glacial retreat(which threatenswater security), increasingextreme weatherevents andwildfires,ecological collapseandmass dying of wildlife.
Images from space
On October 19, 2015, NASA started a website containing daily images of the full sunlit side of Earth athttps://epic.gsfc.nasa.gov/.The images are taken from theDeep Space Climate Observatory(DSCOVR) and show Earth as it rotates during a day.[61]
-
Earth's night-side upper atmosphere appearing from the bottom as bands ofafterglowilluminating thetropospherein orange with silhouettes of clouds, and thestratospherein white and blue. Next themesosphere(pink area) extends to the orange and faintly green line of the lowestairglow,at about one hundred kilometers at theedge of spaceand the lower edge of thethermosphere(invisible). Continuing with green and red bands ofauroraestreching over several hundred kilometers.
-
Limb view of Earth's atmosphere. Colors roughly denote the layers of the atmosphere.
-
This image shows the Moon at the centre, with the limb of Earth near the bottom transitioning into the orange-colored troposphere. The troposphere ends abruptly at the tropopause, which appears in the image as the sharp boundary between the orange- and blue-colored atmosphere. The silvery-bluenoctilucent cloudsextend far above Earth's troposphere.
See also
- Aerial perspective
- Air (classical element)
- Biosphere
- Hydrosphere
- Lithosphere
- Airglow
- Airshed
- Atmospheric dispersion modeling
- Atmospheric electricity
- Climate system
- COSPAR international reference atmosphere(CIRA)
- Environmental effects of aviation
- Global dimming
- Instrumental temperature record
- Reference atmospheric model
References
- ^"Gateway to Astonaut Photos of Earth".NASA.Retrieved2018-01-29.
- ^abCox, Arthur N., ed. (2000),Allen's Astrophysical Quantities(Fourth ed.), AIP Press, pp. 258–259,ISBN0-387-98746-0,which rounds N2and O2to four significant digits without affecting the total because 0.004% was removed from N2and added to O2.It includes 20 constituents.
- ^"What Is... Earth's Atmosphere? - NASA".2024-05-13.Retrieved2024-06-18.
- ^Lide, David R.Handbook of Chemistry and Physics.Boca Raton, FL: CRC, 1996: 14–17
- ^Vázquez, M.; Hanslmeier, A. (2006)."Historical Introduction".Ultraviolet Radiation in the Solar System.Astrophysics and Space Science Library. Vol. 331. Springer Science & Business Media. p. 17.Bibcode:2005ASSL..331.....V.doi:10.1007/1-4020-3730-9_1.ISBN978-1-4020-3730-6.
- ^ab"Trends in Atmospheric Carbon Dioxide",Global Greenhouse Gas Reference Network, NOAA,2019,retrieved2019-05-31
- ^ab"Trends in Atmospheric Methane",Global Greenhouse Gas Reference Network, NOAA,2019,retrieved2019-05-31
- ^abHaynes, H. M., ed. (2016–2017),CRC Handbook of Chemistry and Physics(97th ed.), CRC Press, p. 14-3,ISBN978-1-4987-5428-6,which citesAllen's Astrophysical Quantitiesbut includes only ten of its largest constituents.
- ^abWallace, John M. and Peter V. Hobbs.Atmospheric Science: An Introductory SurveyArchived2018-07-28 at theWayback Machine.Elsevier. Second Edition, 2006.ISBN978-0-12-732951-2.Chapter 1
- ^"Trace Gases".Ace.mmu.ac.uk. Archived fromthe originalon 9 October 2010.Retrieved2010-10-16.
- ^National Aeronautics and Space Administration (1976),U.S. Standard Atmosphere, 1976(PDF),p. 3
- ^Allen, C. W. (1976),Astrophysical Quantities(Third ed.), Athlone Press, p. 119,ISBN0-485-11150-0
- ^Two recent reliable sources cited here have total atmospheric compositions, including trace molecules, that exceed 100%. They areAllen's Astrophysical Quantities[2](2000, 100.001241343%) andCRC Handbook of Chemistry and Physics[8](2016–2017, 100.004667%), which citesAllen's Astrophysical Quantities.Both are used as references in this article. Both exceed 100% because their CO2values were increased to 345 ppmv, without changing their other constituents to compensate. This is made worse by the April 2019 CO2value, which is 413.32 ppmv.[6]Although minor, the January 2019 value forCH4is 1866.1 ppbv (parts per billion).[7]Two older reliable sources have dry atmospheric compositions, including trace molecules, that total less than 100%:U.S. Standard Atmosphere, 1976[11](99.9997147%); andAstrophysical Quantities[12](1976, 99.9999357%).
- ^"Vital signs: Carbon Dioxide".NASA Climate.April 2022.Retrieved16 May2022.
- ^"Vital signs: Methane".NASA Climate.April 2022.Retrieved2 Feb2024.
- ^Detlev Möller:Luft: Chemie, Physik, Biologie, Reinhaltung, Recht.Walter de Gruyter, 2003,ISBN3-11-016431-0,S. 173.(View in Google Books).
- ^Yunus Çengel.Termodinamica e trasmissione del calore.
- ^"Air - Molecular Weight and Composition".www.engineeringtoolbox.com.Retrieved2021-04-27.
- ^"Air Composition".The Engineering ToolBox.Retrieved2017-07-04.
The composition of air is unchanged until elevation of approximately 10.000 m
- ^Zell, Holly (2015-03-02)."Earth's Upper Atmosphere".NASA.Retrieved2017-02-20.
- ^"Exosphere - overview".UCAR. 2011. Archived fromthe originalon 17 May 2017.RetrievedApril 19,2015.
- ^abRandy Russell (2008)."The Thermosphere".Retrieved2013-10-18.
- ^ab"The height of the tropopause".Das.uwyo.edu.Retrieved2012-04-18.
- ^abc"Exosphere - overview".UCAR. 2011.Archivedfrom the original on 17 May 2017.RetrievedApril 19,2015.
- ^"Earth's Atmospheric Layers".January 22, 2013.
- ^David C. Catling and Kevin J. Zahnle,The Planetary Air Leak,Scientific American,May 2009, p. 26 (accessed 25 July 2012)
- ^Ahrens, C. Donald.Essentials of Meteorology.Published by Thomson Brooks/Cole, 2005.
- ^States, Robert J.; Gardner, Chester S. (January 2000)."Thermal Structure of the Mesopause Region (80–105 km) at 40°N Latitude. Part I: Seasonal Variations".Journal of the Atmospheric Sciences.57(1): 66–77.Bibcode:2000JAtS...57...66S.doi:10.1175/1520-0469(2000)057<0066:TSOTMR>2.0.CO;2.
- ^Joe Buchdahl."Atmosphere, Climate & Environment Information Programme".Ace.mmu.ac.uk. Archived fromthe originalon 2010-07-01.Retrieved2012-04-18.
- ^Journal of the Atmospheric Sciences (1993)."stratopause".Archived fromthe originalon 2013-10-19.Retrieved2013-10-18.
- ^Barry, R.G.; Chorley, R.J. (1971).Atmosphere, Weather and Climate.London: Menthuen & Co Ltd. p.65.ISBN9780416079401.
- ^Tyson, P.D.; Preston-Whyte, R.A. (2013).The Weather and Climate of Southern Africa(2nd ed.). Oxford:Oxford University Press.p. 4.
- ^"Troposphere".Concise Encyclopedia of Science & Technology.McGraw-Hill.1984.
It contains about four-fifths of the mass of the whole atmosphere.
- ^"homosphere– AMS Glossary ".Amsglossary.allenpress.com.Archivedfrom the original on 14 September 2010.Retrieved2010-10-16.
- ^Anne Marie Helmenstine, PhD (June 16, 2018)."The 4 Most Abundant Gases in Earth's Atmosphere".
- ^"Earth's Atmosphere".Archived fromthe originalon 2009-06-14.
- ^"NASA – Earth Fact Sheet".Nssdc.gsfc.nasa.gov.Archivedfrom the original on 30 October 2010.Retrieved2010-10-16.
- ^"Global Surface Temperature Anomalies".Archived fromthe originalon 2009-03-03.
- ^"Earth's Radiation Balance and Oceanic Heat Fluxes".Archived fromthe originalon 2005-03-03.
- ^"Coupled Model Intercomparison Project Control Run"(PDF).Archived fromthe original(PDF)on 2008-05-28.
- ^Geometric altitude vs. temperature, pressure, density, and the speed of sound derived from the 1962 U.S. Standard Atmosphere.
- ^Trenberth, Kevin E.; Smith, Lesley (1970-01-01). "The Mass of the Atmosphere: A Constraint on Global Analyses".Journal of Climate.18(6): 864.Bibcode:2005JCli...18..864T.CiteSeerX10.1.1.727.6573.doi:10.1175/JCLI-3299.1.S2CID16754900.
- ^Lutgens, Frederick K. and Edward J. Tarbuck (1995)The Atmosphere,Prentice Hall, 6th ed., pp. 14–17,ISBN0-13-350612-6
- ^"Atmospheric Temperature Trends, 1979–2005: Image of the Day".Earthobservatory.nasa.gov. 2000-01-01.Retrieved2014-06-10.
- ^Holman, Jack P. (2002).Heat transfer(9th ed.). New York, NY: McGraw-Hill Companies, Inc. pp. 600–606.ISBN9780072406559.OCLC46959719.
- ^Bergman, Theodore L.; Lavine, Adrienne S.; Incropera, Frank P.; DeWitt, David P. (2007).Fundamentals of heat and mass transfer(6th ed.). Hoboken, NJ: John Wiley and Sons, Inc. pp. 941–950.ISBN9780471457282.OCLC62532755.
- ^"Absorption / reflection of sunlight".Understanding Global Change.Retrieved2023-06-13.
- ^"The Atmospheric Window".National Oceanic and Atmospheric Administration.Retrieved2023-06-13.
- ^St. Fleur, Nicholas (19 May 2017)."Spotting Mysterious Twinkles on Earth From a Million Miles Away".The New York Times.Retrieved20 May2017.
- ^Marshak, Alexander; Várnai, Tamás; Kostinski, Alexander (15 May 2017)."Terrestrial glint seen from deep space: oriented ice crystals detected from the Lagrangian point".Geophysical Research Letters.44(10): 5197.Bibcode:2017GeoRL..44.5197M.doi:10.1002/2017GL073248.hdl:11603/13118.S2CID109930589.
- ^Edlén, Bengt (1966). "The refractive index of air".Metrologia.2(2): 71–80.Bibcode:1966Metro...2...71E.doi:10.1088/0026-1394/2/2/002.
- ^abZahnle, K.;Schaefer, L.;Fegley, B. (2010)."Earth's Earliest Atmospheres".Cold Spring Harbor Perspectives in Biology.2(10): a004895.doi:10.1101/cshperspect.a004895.PMC2944365.PMID20573713.
- ^B. Windley:The Evolving Continents.Wiley Press, New York 1984
- ^J. Schopf:Earth's Earliest Biosphere: Its Origin and Evolution.Princeton University Press, Princeton, N.J., 1983
- ^ Timothy W. Lyons, Christopher T. Reinhard & Noah J. Planavsky (2014). "Atmospheric oxygenation three billion years ago".Nature.506(7488): 307–15.Bibcode:2014Natur.506..307L.doi:10.1038/nature13068.PMID24553238.S2CID4443958.
- ^Martin, Daniel; McKenna, Helen; Livina, Valerie (2016)."The human physiological impact of global deoxygenation".The Journal of Physiological Sciences.67(1): 97–106.doi:10.1007/s12576-016-0501-0.ISSN1880-6546.PMC5138252.PMID27848144.
- ^Graph: Atmospheric Oxygen and CO2 vs Time
- ^Christopher R. Scotese,Back to Earth History: Summary Chart for the Precambrian,Paleomar Project
- ^Starting from[1]Pollution – Definition from the Merriam-Webster Online Dictionary
- ^IPCC(2021)."Summary for Policymakers"(PDF).IPCC AR6 WG1.pp. 4–5. Archived fromthe original(PDF)on 2021-08-11.Retrieved2021-11-20.
- ^Northon, Karen (2015-10-19)."Daily Views of Earth Available on New NASA Website".NASA.Retrieved2015-10-21.
External links
- Buchan, Alexander(1878)..Encyclopædia Britannica.Vol. III (9th ed.). pp. 28–36.
- Interactive global map of current atmospheric and ocean surface conditions.





