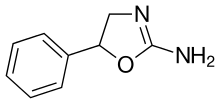
Aminorex
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.164.420 |
| Chemical and physical data | |
| Formula | C9H10N2O |
| Molar mass | 162.192g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Aminorex(Menocil,Apiquel,aminoxaphen,aminoxafen,McN-742) is a weight loss (anorectic)stimulantdrug.It was withdrawn from the market after it was found to causepulmonary hypertension.[2]In the U.S., it is an illegalSchedule Idrug, meaning it has high abuse potential, no accepted medical use, and a poor safety profile.
Aminorex, in the 2-amino-5-aryl oxazoline class, was developed byMcNeil Laboratoriesin 1962.[3]It is closely related to4-methylaminorex.Aminorex has been shown to have locomotor stimulant effects, lying midway betweendextroamphetamineandmethamphetamine.Aminorex effects have been attributed to the release ofcatecholamines.[4]It can be produced as ametaboliteof the worming medicationlevamisole,which is sometimes used as acutting agentof illicitly producedcocaine.[5][6]
Pharmacology
[edit]Aminorex has been found to act as a reuptake inhibitor at the dopamine and norepinephrine transporters, with releasing agent properties at the serotonin transporter.[7]
History
[edit]It was discovered in 1962 byEdward John Hurlburt,[8]and was quickly found in 1963 to have ananorecticeffect inrats.It was introduced as aprescriptionappetite suppressant inGermany,SwitzerlandandAustriain 1965, but was withdrawn in 1972 after it was found to causepulmonary hypertensionin approximately 0.2% of patients, and was linked to a number of deaths.[4][9]
Synthesis
[edit]The synthesis was first reported in astructure-activity relationshipstudy of 2-amino-5-aryl-2-oxazolines, where aminorex was found to be approximately 2.5 times more potent thanD-amphetamine sulfate in inducing anorexia in rats, and was also reported to have CNS stimulant effects.
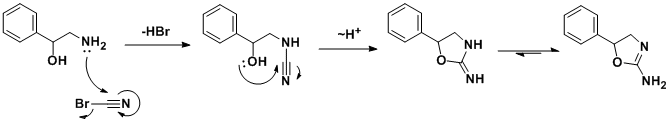
Theracemicsynthesis involves addition/cyclization reaction of 2-amino-1-phenylethanol withcyanogen bromide.[10]A similar synthesis has been also published.[11]In a search for a cheaper synthetic route, a German team developed an alternative route[12]which, by using chiral styrene oxide, allows an enantiopure product.
See also
[edit]- 4-Methylaminorex
- Clominorex
- Cyclazodone
- Fenozolone
- Fluminorex
- Pemoline
- Thozalinone
- List of aminorex analogues
References
[edit]- ^Anvisa(2023-03-31)."RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial"[Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese).Diário Oficial da União(published 2023-04-04).Archivedfrom the original on 2023-08-03.Retrieved2023-08-16.
- ^Gaine SP, Rubin LJ, Kmetzo JJ, Palevsky HI, Traill TA (November 2000)."Recreational use of aminorex and pulmonary hypertension".Chest.118(5): 1496–1497.doi:10.1378/chest.118.5.1496.PMID11083709.Archived fromthe originalon 2013-01-12.
- ^US 3161650,Ireland PG, "2-Amino-5-Aryloxazoline Products", issued 15 December 1964, assigned to Janssen Pharmaceuticals Inc.
- ^abFishman AP (Jan 1991)."Aminorex to fen/phen: an epidemic foretold".Circulation.99(1): 156–161.doi:10.1161/01.CIR.99.1.156.PMID9884392.
- ^Ho EN, Leung DK, Leung GN, Wan TS, Wong AS, Wong CH, et al. (April 2009). "Aminorex and rexamino as metabolites of levamisole in the horse".Analytica Chimica Acta.638(1): 58–68.Bibcode:2009AcAC..638...58H.doi:10.1016/j.aca.2009.02.033.PMID19298880.
- ^Bertol E, Mari F, Milia MG, Politi L, Furlanetto S, Karch SB (July 2011). "Determination of aminorex in human urine samples by GC-MS after use of levamisole".Journal of Pharmaceutical and Biomedical Analysis.55(5): 1186–1189.doi:10.1016/j.jpba.2011.03.039.PMID21531521.
- ^Hofmaier T, Luf A, Seddik A, Stockner T, Holy M, Freissmuth M, et al. (July 2014)."Aminorex, a metabolite of the cocaine adulterant levamisole, exerts amphetamine like actions at monoamine transporters".Neurochemistry International.73(100): 32–41.doi:10.1016/j.neuint.2013.11.010.PMC4077236.PMID24296074.
- ^US 3115494,Albert MG, Ireland PG, "2-amino-5, 6-dihydro-4ii-1, 3-oxazines and a process for their preparation", issued 2 December 1963, assigned to Janssen Pharmaceuticals Inc.
- ^Weigle DS (June 2003)."Pharmacological therapy of obesity: past, present, and future".The Journal of Clinical Endocrinology and Metabolism.88(6): 2462–2469.doi:10.1210/jc.2003-030151.PMID12788841.
- ^Poos GI, Carson JR, Rosenau JD, Roszkowski AP, Kelley NM, Mcgowin J (May 1963). "2-Amino-5-aryl-2-oxazolines. Potent New Anorectic Agents".Journal of Medicinal Chemistry.6(3): 266–272.doi:10.1021/jm00339a011.PMID14185981.
- ^Ueda S, Terauchi H, Yano A, Ido M, Matsumoto M, Kawasaki M (January 2004). "4,5-Disubstituted-1,3-oxazolidin-2-imine derivatives: a new class of orally bioavailable nitric oxide synthase inhibitor".Bioorganic & Medicinal Chemistry Letters.14(2): 313–316.doi:10.1016/j.bmcl.2003.11.010.PMID14698148.
- ^DE 2101424,"2-Amino-5-phenyl-2-oxazoline preparation", assigned to Polska Akademia Nauk Instytut Chemn Organicznej, Warschau
