Atipamezole
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Antisedan, others |
| AHFS/Drugs.com | |
| License data | |
| Routes of administration | Intramuscular |
| Drug class | Reversal agent |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Pharmacokineticdata | |
| Metabolism | Liver |
| Onset of action | Less than 3 min. |
| Eliminationhalf-life | 2.6 hours (dogs) |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard(EPA) | |
| Chemical and physical data | |
| Formula | C14H16N2 |
| Molar mass | 212.296g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Atipamezole,sold under the brand nameAntisedanamong others, is a syntheticα2adrenergic receptorantagonistused for the reversal of the sedative and analgesic effects ofdexmedetomidineandmedetomidinein dogs. Its reversal effect works by competing with the sedative forα2-adrenergic receptorsand displacing them. It is mainly used in veterinary medicine, and while it is only licensed fordogsand forintramuscularuse, it has been usedintravenously,as well as incatsand other animals(intravenous use in cats and dogs is not recommended due to the potential for cardiovascular collapse. This occurs due to profound hypotension caused by reversal of the alpha 1 effects while the reflex bradycardia is still in effect.). There is a low rate of side effects, largely due to atipamezole's high specificity for the α2-adrenergic receptor. Atipamezole has a very quick onset, usually waking an animal up within 5 to 10 minutes.[medical citation needed]
It was originally released in 1996.[7]It is available in as ageneric medication.[8]
Veterinary use
[edit]Atipamezole is a veterinary drug whose prime purpose is to reverse the effects of the sedativedexmedetomidine(as well as itsracemicmixture,medetomidine).[note 1][9][10]It can also be used to reverse the related sedativexylazine.[11]While it reverses both the sedative andanalgesic(pain-relieving) effects of dexmedetomidine, atipamezole may not entirely reverse thecardiovasculardepression that dexmedetomidine causes.[9][12][13]
Atipamezole is licensed in the United States forintramuscular injection(IM) indogs;it is, however, used off-label incats,rabbits,[14]and farm animals such ashorsesandcows,[12]as well as in zoo medicine forreptiles(includingtortoises,turtles,andalligators),armadillos,hippopotamuses,giraffes,okapi,and others.[15][16]It has been givenintravenously(IV),subcutaneously,intraperitoneallyand, inred-eared sliders,intranasally.[17][18]
Atipamezole has also been used as an antidote for various toxicities in dogs. For example, the anti-tickmedicationamitrazis commonly ingested by dogs who eat theiranti-tick collars.[19]Amitraz works by the same mechanism as dexmedetomidine and is thus easily reversed by atipamezole.[20][21]Atipamezole also reverses the hypotension caused bytizanidine(a muscle relaxant) toxicity, and relieves toxicity fromdecongestantssuch asephedrineandpseudoephedrine.[22]
Available forms
[edit]Atipamezole is sold at 5 mg/mL for ease of use: 5 times as much atipamezole as medetomidine is needed for full reversal, and because medetomidine is sold as 1 mg/mL, 1 mL of atipamezole reverses 1 mL of medetomidine.[23]When the enantiomerically pure version of medetomidine (dexmedetomidine) was released, it was sold at 0.5 mg/mL, because it was twice as strong as medetomidine. As such, 1 mL of atipamezole also reverses 1 mL of dexmedetomidine.[10][12]
Specific populations
[edit]Atipamezole is not recommended for animals that are pregnant,lactating,or slated for breeding.[24]
Contraindications
[edit]While there are no absolute contraindications to atipamezole, it is recommended against being given withanticholinergics,as both can cause dramatic increases in heart rate.[11][20]Atipamezole should also not be given too soon after an animal has been given dexmedetomidine mixed withketamineortelazol(tiletamine); because it reverses only the dexmedetomidine, the ketamine or telazol will still be active, and the animal can wake up excited, delirious, and with muscle contractions.[25]Some recommend not using it in dogs sedated with ketamine at all, since they can convulse due to the excitement effect.[26]
Side effects
[edit]Atipamezole's low rate of side effects is due to its high specificity forɑ2-adrenergic receptors;it has very little affinity forɑ1-adrenergic receptorsand no affinity for mostserotonin,muscarinic,anddopamine receptors.[12][27][28]There is occasionalvomiting,hypersalivation,anddiarrhea.It can potentially causeCNSexcitement, which can lead totremors,tachycardia(increased heart rate), andvasodilation.The vasodilation leads to a transient decrease inblood pressure,which (in dogs) increases to normal within 10 minutes.[9]There have been reports of transienthypoxemia.[25]The chance of side effect can be minimized by administering atipamezole slowly.[12]

There is a possibility of the sedation reversing abruptly, leading to nervous, aggressive, or delirious dogs.[9]Such cases are more associated with intravenous administration[29](which has a faster onset than IM administration). The rapid administration of atipamezole leads to sudden displacement of dexmedetomidine from peripheral ɑ2-adrenergic receptors; this can cause a sudden drop in blood pressure, which is followed by a reflex tachycardia and hypertension.[12][26][30]
There have been some cases where intravenous administration of atipamezole lead to death viacardiovascular collapse.This is thought to be combination of sudden hypotension added onto the low heart rate caused by sedatives.[12]
There is some possibility of the animal relapsing into sedation after being given atipamezole, made more likely if the original sedative was given intravenously.[9]
Rats and monkeys have experienced increased sexual activity after being given atipamezole.[31][32]
Overdose
[edit]TheLD50of atipamezole for rats is 44 mg/kg when given subcutaneously. Theminimum lethal dosein dogs is over 5 mg/m2;dogs have tolerated getting ten times the standard dose.[9][33]Signs of overdose include panting, trembling, vomiting, and diarrhea, as well as increased blood levels ofcreatine kinase,aspartate transaminase,andalanine transaminase.Dogs who received atipamezole without first receiving dexmedetomidine have shown no clinical signs other than mild muscle tremors.[9][23]
Pharmacology
[edit]Mechanism of action
[edit]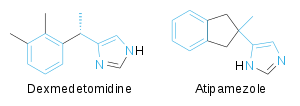
Atipamezole is a competitiveantagonist at ɑ2-adrenergic receptorsthat competes with dexmedetomidine, anɑ2-adrenergic receptors agonist.It does not directly interact with dexmedetomidine;[34]rather, their structural similarity allows atipamezole to easily compete for receptor binding sites.[12]
Atipamezole reverses analgesia by blockingnorepinephrinefeedback inhibition on nociceptors.[12][31]
| Site | Ki(nM) | Species | Ref |
|---|---|---|---|
| α1 | 3160 | Human | [35] |
| α2A | 1.9 | Human | [36] |
| α2B | 2.2 | Human | [36] |
| α2C | 4.2 | Human | [36] |
| TheKirefers to a drug's affinity for a receptor. The smaller
the Ki,the higher the affinity for that receptor. | |||
Pharmacokinetics
[edit]Out of the three ɑ2-antagonists commonly used in veterinary medicine (atipamezole,yohimbine,and tolazine), atipamezole shows the highest preference for ɑ2- over ɑ1-receptors, binding to them with a ratio of 8526:1.[12]It shows no preference for a particular ɑ2-receptor subtype.[31]
Atipamezole has a rapid onset: it reverses the decreased heart rate caused by sedation within three minutes. The animal usually begins waking up within 5–10 minutes. In a study of over 100 dogs, more than half could stand up within 5 minutes, and 96% could stand up within 15. Atipamezole reaches maximum serum concentration within 10 minutes of IM administration.[9]Atipamezole is distributed extensively to the tissues; at a particular time, concentrations in the brain reach two to three times the concentration in the plasma.[27]
Atipamezole undergoes heavyfirst-pass metabolismin the liver,[27]which includes theglucuronidationat nitrogen during.[37]Metabolites are mostly excreted in the urine.[38]
The elimination half-life is 2.6 hours in dogs and 1.3 hours rats.[9][20]
Research
[edit]Atipamezole's effects oncognitive functionhave been studied in rats and in humans. While low doses in rats improved alertness, selective attention, learning, and recall, higher doses generally impaired cognitive function (most likely due to norepinephrine overactivity).[31]In rats, it has also been shown to improve cognitive function decreased bystrokesor brain lesions.[20]Studies in humans have found it to increase focus but decrease multitasking abilities.[27]Atipamezole has also been researched in humans as a potentialanti-Parkinsonian.[27]
Because atipamezole increases sexual activity in monkeys, there have been claims of its potential to treat erectile dysfunction.[32]
Notes
[edit]- ^Because dexmedetomidine is the only pharmacologically active component of medetomidine, they will both be referred to asdexmedetomidinefrom here on out.
References
[edit]- ^"Antisedan Product information".health-products.canada.ca.24 March 2011.Retrieved5 April2024.
- ^"Antisedan- atipamezole hydrochloride injection, solution".DailyMed.18 June 2020.Retrieved5 April2024.
- ^"Contrased- atipamezole hydrochloride injection, solution".DailyMed.1 March 2024.Retrieved5 April2024.
- ^"Cropamezole- atipamezole hydrochloride injection, solution".DailyMed.26 December 2023.Retrieved5 April2024.
- ^"Revertased- atipamezole hydrochloride injection, solution".DailyMed.14 September 2023.Retrieved5 April2024.
- ^"Revertidine- atipamezole hydrochloride injection, solution".DailyMed.12 January 2023.Retrieved5 April2024.
- ^Ettinger SJ, Feldman EC (2009).Textbook of Veterinary Internal Medicine - eBook(7th ed.). Elsevier Health Sciences. p. 61.ISBN978-1-4377-0282-8.
- ^"Atipamezole".Drugs.com.Retrieved7 August2019.
- ^abcdefghi"Antisedan for Animal Use".Drugs.com.Retrieved24 February2018.
- ^abCote 2010,p.1623.
- ^abPapich MG (2010).Saunders Handbook of Veterinary Drugs – E-Book: Small and Large Animal.Elsevier Health Sciences. p. 56.ISBN978-1-4377-0192-0.
- ^abcdefghijRiviere JE, Papich MG (2009).Veterinary Pharmacology and Therapeutics(illustrated ed.). John Wiley & Sons. pp. 352–355.ISBN978-0-8138-2061-3.
- ^Talke P, Harper D, Traber L, Richardson CR, Traber D (February 1999). "Reversal of medetomidine induced sedation by atipamezole in sheep: Effects on organ blood".Anesthesia & Analgesia.88(2S): 391S.doi:10.1097/00000539-199902001-00388.ISSN0003-2999.
- ^Kim MS, Jeong SM, Park JH, Nam TC, Seo KM (October 2004)."Reversal of medetomidine-ketamine combination anesthesia in rabbits by atipamezole".Experimental Animals.53(5): 423–428.doi:10.1538/expanim.53.423.PMID15516790.
- ^Heaton-Jones TG, Ko JC, Heaton-Jones DL (March 2002). "Evaluation of medetomidine-ketamine anesthesia with atipamezole reversal in American alligators (Alligator mississippiensis)".Journal of Zoo and Wildlife Medicine.33(1): 36–44.doi:10.1638/1042-7260(2002)033[0036:EOMKAW]2.0.CO;2.PMID12216791.S2CID25527884.
- ^Miller RE, Fowler ME (2014).Fowler's Zoo and Wild Animal Medicine, Volume 8 – E-Book(revised ed.). Elsevier Health Sciences. pp. 29, 358, 587, 605.ISBN978-1-4557-7399-2.
- ^Mader DR, Divers SJ (2013).Current Therapy in Reptile Medicine and Surgery - E-Book.Elsevier Health Sciences. pp. 143, 387.ISBN978-0-323-24293-6.
- ^Wang-Fischer Y (2008).Manual of Stroke Models in Rats.CRC Press. p. 65.ISBN978-1-4200-0952-1.
- ^DeClementi C (2007). "Chapter 91: Prevention and treatment of poisoning". In Gupta R (ed.).Veterinary Toxicology.Oxford: Academic Press. pp. 1139–1158.doi:10.1016/B978-012370467-2/50188-7.ISBN978-0-12-370467-2.
- ^abcdBahri L (May 2008)."Pharm Profile: Atipamezole".Compendium.30(5).
- ^Gupta RC (2007). "Chapter 46: Amitraz".Veterinary Toxicology.Oxford: Academic Press. pp. 514–517.doi:10.1016/B978-012370467-2/50143-7.ISBN978-0-12-370467-2.
- ^Cote 2010,pp.126,285.
- ^abClarke KW, Trim CM (2013).Veterinary Anaesthesia E-Book(11th ed.). Elsevier Health Sciences. p. 91.doi:10.1016/B978(inactive 13 September 2024).ISBN978-0-7020-5423-5.
{{cite book}}:CS1 maint: DOI inactive as of September 2024 (link) - ^L.S.A., List of C.F.R. Sections Affected.National Archives of the United States. 2004. p. 221.ISBN978-0-16-072065-9.
- ^abSchenck P (2009).Saunders Comprehensive Review of the NAVLE – E-Book.Elsevier Health Sciences. p. 402.ISBN978-1-4377-1448-7.
- ^abDugdale A (2011).Veterinary Anaesthesia: Principles to Practice.John Wiley & Sons. pp. 257, 368.ISBN978-1-118-27933-5.
- ^abcdePertovaara A, Haapalinna A, Sirviö J, Virtanen R (1 September 2005)."Pharmacological properties, central nervous system effects, and potential therapeutic applications of atipamezole, a selective alpha2-adrenoceptor antagonist".CNS Drug Reviews.11(3): 273–288.doi:10.1111/j.1527-3458.2005.tb00047.x.PMC6741735.PMID16389294.
- ^Sawyer D (2008).The Practice of Veterinary Anesthesia: Small Animals, Birds, Fish and Reptiles.Manson Series. CRC Press. p. 42.ISBN978-1-59161-034-2.
- ^Fish 2008,p.371.
- ^Divers SJ, Mader DR (2005).Reptile Medicine and Surgery - E-Book(2 ed.). Elsevier Health Sciences. p. 444.ISBN978-1-4160-6477-0.
- ^abcdFish 2008,pp.53–54.
- ^abAnnual Reports in Medicinal Chemistry.Vol. 34. Academic Press. 1999. p. 78.ISBN978-0-08-058378-5.
- ^"Safety Data Sheet"(PDF).Zoetis. 20 March 2017.
- ^Grant D (2006).Pain Management in Small Animals.Elsevier Health Sciences. pp. 191, 199.ISBN978-0-7506-8812-3.
- ^Blaxall HS, Murphy TJ, Baker JC, Ray C, Bylund DB (October 1991)."Characterization of the alpha-2C adrenergic receptor subtype in the opossum kidney and in the OK cell line".The Journal of Pharmacology and Experimental Therapeutics.259(1): 323–329.PMID1656026.
- ^abcVacher B, Funes P, Chopin P, Cussac D, Heusler P, Tourette A, et al. (October 2010). "Rigid analogues of the α2-adrenergic blocker atipamezole: small changes, big consequences".Journal of Medicinal Chemistry.53(19): 6986–6995.doi:10.1021/jm1006269.PMID20809632.
- ^Kaivosaari S, Salonen JS, Taskinen J (March 2002)."N-Glucuronidation of some 4-arylalkyl-1H-imidazoles by rat, dog, and human liver microsomes".Drug Metabolism and Disposition.30(3): 295–300.doi:10.1124/dmd.30.3.295.PMID11854148.
- ^Peterson ME, Kutzler M (2010).Small Animal Pediatrics – E-Book: The First 12 Months of Life.Elsevier Health Sciences. p. 226.ISBN978-1-4377-0195-1.
Further reading
[edit]- Cote E (2010).Clinical Veterinary Advisor – E-Book: Dogs and Cats(2nd, revised ed.). Elsevier Health Sciences.ISBN978-0-323-06876-5.
- Fish RE (2008).Anesthesia and Analgesia in Laboratory Animals.American College of Laboratory Animal Medicine series. Academic Press.ISBN978-0-12-373898-1.
External links
[edit] Media related toAtipamezoleat Wikimedia Commons
Media related toAtipamezoleat Wikimedia Commons
