Azo dye

Azo dyesareorganic compoundsbearing thefunctional groupR−N=N−R′, in which R and R′ are usuallyaryland substituted aryl groups. They are a commercially important family ofazo compounds,i.e. compounds containing the C-N=N-C linkage.[1]Azo dyes are syntheticdyesand do not occur naturally.[2][3]Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60-70% of all dyes used infoodandtextileindustries.[3]Azo dyes are widely used to treattextiles,leather articles,and some foods. Chemically related derivatives of azo dyes includeazo pigments,which are insoluble in water and other solvents.[4][5]
Classes
[edit]Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes,metal-complex dyes,reactive dyes,andsubstantive dyes.Also called direct dyes, substantive dyes are employed for cellulose-based textiles, which includes cotton. The dyes bind to the textile by non-electrostatic forces. In another classification, azo dyes can be classified according to the number of azo groups.

Physical properties, structure, and bonding
[edit]As a consequence of π-delocalization,aryl azo compounds have vivid colors, especially reds, oranges, and yellows. An example isDisperse Orange 1.Some azo compounds, e.g.,methyl orange,are used asacid-base indicators.MostDVD-R/+Rand someCD-Rdiscs use blue azo dye as the recording layer.

Azo dyes are solids. Most are salts, the colored component being the anion usually, although some cationic azo dyes are known. The anionic character of most dyes arises from the presence of 1-3 sulfonic acid groups, which are fully ionized at the pH of the dyed article:
- RSO3H → RSO3−+ H+
Most proteins are cationic, thus dyeing of leather and wool corresponds to anion exchangereaction. The anionic dye adheres to these articles through electrostatic forces. Cationic azo dyes typically containquaternary ammoniumcenters.
Preparation
[edit]Most azo dyes are prepared byazo coupling,which entails anelectrophilic substitution reactionof anaryl diazonium cationwith another compound, the coupling partner. Generally, coupling partners are other aromatic compounds with electron-donating groups:[7]
- ArN+
2+ Ar′H → ArN=NAr′ + H+
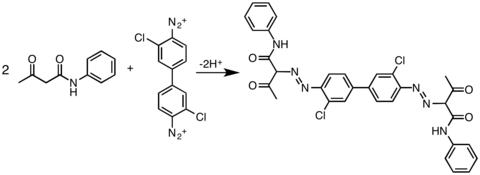
In practice, acetoacetic amide are widely used as coupling partners:
- ArN+
2+ Ar′NHC(O)CH2C(O)Me → ArN=NCH(C(O)Me)(C(O)NHAr′) + H+
Azo dyes are also prepared by the condensation of nitrated aromatic compounds withanilinesfollowed by reduction of the resultingazoxyintermediate:
- ArNO2+ Ar′NH2→ ArN(O)=NAr′ + H2O
- ArN(O)=NAr′ + C6H12O6→ ArN=NAr′ + C6H10O6+ H2O
For textile dying, a typical nitro coupling partner would bedisodium 4,4′-dinitrostilbene-2,2′-disulfonate.Typical aniline partners are shown below. Since anilines are prepared from nitro compounds, some azo dyes are produced by partial reduction of aromatic nitro compounds.[5]
Many azo dyes are produced by reactions from pre-existing azo compounds. Typical reactions include metal complexation and acylation.
- Illustrative azo dyes or their precursors
-
Direct Brown 78
-
Basic Red 18,a cationic azo dye
Azo pigments
[edit]Azo pigments are similar in chemical structure to azo dyes, but they lack solubilizing groups. Because they are practically insoluble in all solvents, they are not readily purified, and thus require highly purified precursors.
Azo pigments are important in a variety of plastics, rubbers, andpaints(including artist's paints). They have excellent coloring properties, mainly in the yellow to red range, as well as goodlightfastness.The lightfastness depends not only on the properties of the organic azo compound, but also on the way they have been absorbed on the pigment carrier.
Azo pigments amongst food pigments are the oldest and also the most widely used. They were discovered byPeter Griessin 1858.[8]
Biodegradation
[edit]In order for dyes to be useful, they must possess a high degree of chemical and photolytic stability. As a result of this stability,photolysisis not considered to be a degradation pathway for azo dyes. In order to prolong the lifetime of products dyed with azo dyes, it is essential to ensure stability against microbial attack, and tests have shown that azo dyes biodegrade negligibly in short term tests under aerobic conditions. Under anaerobic conditions, however, discoloration may be observed as a consequence of biodegradation.[9]
Safety and regulation
[edit]Many azo pigments are non-toxic, although some, such asdinitroanilineorange,ortho-nitroanilineorange, or pigment orange 1, 2, and 5 aremutagenicandcarcinogenic.[10][11]
Azo dyes derived frombenzidinearecarcinogens;exposure to them has classically been associated withbladder cancer.[12]Accordingly, the production of benzidine azo dyes was discontinued in the 1980s in many western countries.[5]
European regulation
[edit]Certain azo dyes degrade under reductive conditions to release any of a group of definedaromatic amines.Since September 2003, the European Union has banned the manufacture or sale of consumer goods which contain the listed amines. Since only a small number of dyes produced those amines, relatively few products were actually affected.[4]
See also
[edit]References
[edit]- ^IUPAC,Compendium of Chemical Terminology,2nd ed. (the "Gold Book" ) (1997). Online corrected version: (2006–) "azo compounds".doi:10.1351/goldbook.A00560
- ^Benkhaya, Said; M'rabet, Souad; El Harfi, Ahmed (31 January 2020)."Classifications, properties, recent synthesis and applications of azo dyes".Heliyon.6(1): e03271.Bibcode:2020Heliy...603271B.doi:10.1016/j.heliyon.2020.e03271.ISSN2405-8440.PMC7002841.PMID32042981.
- ^ab"Azo dyes".Food-Info.net.Archivedfrom the original on 29 November 2022.Retrieved29 November2022.
- ^abPüntener, A.; Page, C."European Ban on Certain Azo Dyes"(PDF).Quality and Environment, TFL.Archived fromthe original(PDF)on 13 August 2012.
- ^abcHunger, Klaus; Mischke, Peter; Rieper, Wolfgang; et al. (2000). "Azo Dyes".Ullmann's Encyclopedia of Industrial Chemistry.doi:10.1002/14356007.a03_245.ISBN3-527-30673-0.
- ^Paola Gilli; Valerio Bertolasi; Loretta Pretto; et al. (2002). "The Nature of Solid-State N−H···O/O−H···N Tautomeric Competition in Resonant Systems. Intramolecular Proton Transfer in Low-Barrier Hydrogen Bonds Formed by the ···OC−CN−NH··· ⇄ ···HO−CC−NN··· Ketohydrazone−Azoenol System. A Variable-Temperature X-ray Crystallographic and DFT Computational Study".J. Am. Chem. Soc.124(45): 13554–13567.doi:10.1021/ja020589x.PMID12418911.
- ^H. T. Clarke; W. R. Kirner (1941)."Methyl Red".Organic Syntheses;Collected Volumes,vol. 1, p. 374.
- ^Diacu, E. (2016)."Colors: Properties and Determination of Synthetic Pigments".In Caballero, Benjamin; Finglas, Paul M.; Toldrá, Fidel (eds.).Encyclopedia of Food and Health.Oxford: Academic Press. pp. 284–290.doi:10.1016/B978-0-12-384947-2.00191-4.ISBN9780123849533.Retrieved29 November2022.
- ^Bafana, Amit; Devi, Sivanesan Saravana; Chakrabarti, Tapan (28 September 2011). "Azo dyes: past, present and the future".Environmental Reviews.19(NA): 350–371.doi:10.1139/a11-018.ISSN1181-8700.
- ^"Health & Safety in the Arts, A Searchable Database of Health & Safety Information for Artists".City of Tucson. Archived fromthe originalon 10 May 2009.
- ^Eva Engel; Heidi Ulrich; Rudolf Vasold; et al. (2008). "Azo Pigments and a Basal Cell Carcinoma at the Thumb".Dermatology.216(1): 76–80.doi:10.1159/000109363.PMID18032904.S2CID34959909.
- ^Golka, K.; Kopps, S.; Myslak, Z. W. (June 2004). "Carcinogenicity of azo colorants: influence of solubility and bioavailability".Toxicology Letters.151(1): 203–10.doi:10.1016/j.toxlet.2003.11.016.PMID15177655.