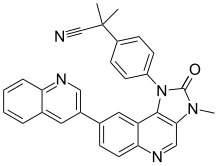
Dactolisib
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methyl-2-{4-[3-methyl-2-oxo-8-(quinolin-3-yl)-2,3-dihydro-1H-imidazo[4,5-c]quinolin-1-yl]phenyl}propanenitrile | |
| Other names
NVP-BEZ235; BEZ-235; RTB101
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C30H23N5O | |
| Molar mass | 469.548g·mol−1 |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Dactolisib(codenamedNVP-BEZ235andBEZ-235,also known asRTB101) is animidazoquinolinederivative acting as aPI3K inhibitor.[1]It also inhibitsmTOR.[2]It is being investigated as a possible cancer treatment.[3]
It has been shown to be toxic toWaldenström's macroglobulinemiacells.[4]
It was the first PI3K inhibitor to enter clinical trials, in 2006.[5]
A phase IB/II clinical trial for locally advanced or metastaticHER2 negative breast cancerhas completed.[6]
A phase II clinical trial for advancedpancreatic neuroendocrine tumors(pNET) had initially reported results, but was later terminated because insufficient normal tissue tolerance to the drug.[7] A phase I clinical trial of BEZ235 in patients with advanced renal cell carcinoma had to be terminated prematurely due to toxicity and a lack of clinical efficacy.[8] Another Phase Ib study on patients with various solid cancers found severe normal tissue toxicity as well when BEZ235/Dactolisib was administered in combination with the mTOR inhibitor Everolimus. The authors concluded that the combination of both drugs demonstrated limited efficacy and tolerance. BEZ235 systemic exposure increased in a dose-proportional manner while oral bioavailability was quite low, which may be related to gastrointestinal-specific toxicity.[9] A phase I study of BEZ-235 to treat acute lymphoid leukaemia was initiated in 2012, but no results were published since then.[10]
Aphase 2arandomized, placebo-controlled clinical trial published in 2018 showed thateverolimusin combination with dactolisib decreased the rate of reported infections in an elderly population.[11]
References
[edit]- ^Liu, TJ; Koul, D; LaFortune, T; Tiao, N; Shen, RJ; Maira, SM; Garcia-Echevrria, C; Yung, WKA (11 August 2009)."NVP-BEZ235, a Novel Dual Phosphatidylinositol 3-kinase/Mammalian Target of Rapamycin Inhibitor, Elicits Multifaceted Antitumor Activities in Human Gliomas".Molecular Cancer Therapeutics.8(8): 2204–10.doi:10.1158/1535-7163.MCT-09-0160.PMC2752877.PMID19671762.
- ^Awasthi, N; Yen, PL; Schwarz, MA; Schwarz, RE (March 2012). "The Efficacy of a Novel, Dual PI3K/mTOR Inhibitor NVP-BEZ235 to Enhance Chemotherapy and Antiangiogenic Response in Pancreatic Cancer".Journal of Cellular Biochemistry.113(3): 784–91.doi:10.1002/jcb.23405.PMID22020918.S2CID23005922.
- ^Maira, SM; Stauffer, F; Schnell, C; García-Echeverría, C (1 February 2009). "PI3K Inhibitors for Cancer Treatment: Where Do We Stand?".Biochemical Society Transactions.37(1): 265–72.doi:10.1042/BST0370265.PMID19143644.
- ^Sacco, A; Roccaro, A; Ghobrial, IM (November 2010)."Role of Dual PI3/Akt and mTOR Inhibition in Waldenström's Macroglobulinemia".Oncotarget.1(7): 578–82.doi:10.18632/oncotarget.192.PMC3248138.PMID21317453.
- ^"A Phase I/II Study of BEZ235 in Patients with Advanced Solid Malignancies Enriched by Patients with Advanced Breast Cancer".ClinicalTrials.gov.Retrieved16 July2016.
- ^Phase Ib/II Trial of BEZ235 With Paclitaxel in Patients With HER2 Negative, Locally Advanced or Metastatic Breast Cancer
- ^BEZ235 Phase II Trial in Patients With Advanced Pancreatic Neuroendocrine Tumors (pNET) After Failure of mTOR Inhibitor Therapy.
- ^Pongas, G.; Fojo, T. (2016)."BEZ235: When Promising Science Meets Clinical Reality".The Oncologist.21(9): 1033–1034.doi:10.1634/theoncologist.2016-0243.PMC5016067.PMID27566248.
- ^"A Phase Ib Study of the Dual PI3K/mTOR Inhibitor Dactolisib(BEZ235) Combined with Everolimus in Patients with AdvancedSolid Malignancies".Target Oncology.Retrieved29 March2017.
- ^"A Phase I, Dose-finding Study of BEZ235 in Adult Patients With Relapsed or Refractory Acute Leukemia".clinicatrials.gov.Retrieved29 July2020.
- ^Zhavoronkov A(2020)."Geroprotective and senoremediative strategies to reduce the comorbidity, infection rates, severity, and lethality in gerophilic and gerolavic infections".Aging.12(8): 6492–6510.doi:10.18632/aging.102988.PMC7202545.PMID32229705.
