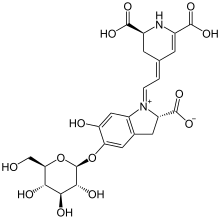
Betanin
| |
| |
| Names | |
|---|---|
| IUPAC name
(2S)-1-{2-[(2S)-2,6-dicarboxy-2,3-dihydropyridin-4(1H)-ylidene]ethylidene}-5-(β-d-glucopyranosyloxy)-6-hydroxy-2,3-dihydro-1H-indol-1-ium-2-carboxylate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.753 |
| E number | E162(colours) |
| KEGG | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C24H26N2O13 | |
| Molar mass | 550.47 g/mol |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Betanin,orbeetroot red,is a redglycosidicfood dyeobtained frombeets;itsaglycone,obtained by hydrolyzing theglucosemolecule, is betanidin. As afood additive,itsE numberis E162.[1]As a food additive, betanin has no safety concerns.[1]
The color of betanin depends onpH;between pH four and five, it is bright bluish-red, becoming blue-violet as the pH increases. Once the pH reaches alkaline levels, betanin degrades byhydrolysis,resulting in a yellow-brown color.[citation needed]
Betanin is abetalainpigment, together with isobetanin and other betacyanins.[2]
Sources
[edit]Betanin is usually obtained from the extract of beet juice; the concentration of betanin in red beet can reach 300–600 mg/kg. Other dietary sources of betanin and other betalains include theOpuntiacactus,Swiss chard,and the leaves of some strains ofamaranth.[citation needed]
Uses
[edit]The most common uses of betanins are in coloringice creamandpowderedsoft drinkbeverages; other uses are in some sugarconfectionery,e.g.fondants,sugar strands, sugar coatings, and fruit or cream fillings. In hot processed candies, it can be used if added at the final part of the processing. Betanin is also used in soups as well astomatoandbaconproducts. Betanin has "not been implicated as causing clinical food allergy when used as a coloring agent".[3]
Betanin has also shown to have antimicrobial activity and can be used as a natural antimicrobial agent infood preservation.[4]
Degradation and stability
[edit]Betanin degrades when subjected tolight,heat, andoxygen;therefore, it is used in frozen products, products with short shelf life, or products sold in dry state. Betanin can survivepasteurizationwhen in products with highsugarcontent. Its sensitivity to oxygen is highest in products with a high water content and/or containing metal cations (e.g.ironandcopper);antioxidantslikeascorbic acidandsequestrantscan slow this process down, together with suitable packaging. In dry form betanin is stable in the presence of oxygen.[5]
Safety
[edit]Use of betanin and other betacyanins as food coloring has a long history in Europe.[1] A scientific panel for theEuropean Food Safety Authorityfound that acute or chronic toxicity andcarcinogenicitystudies were too limited to determine that these food colorants were unsafe.[1]The panel also concluded that the available toxicological database could not provide data to assign anacceptable daily intakelevel for beetroot red.[1]
See also
[edit]References
[edit]- ^abcdeEFSA Panel on Food Additives and Nutrient Sources added to Food (9 December 2015)."Scientific Opinion on the re-evaluation of beetroot red (E 162) as a food additive".EFSA Journal.13(12): 4318.doi:10.2903/j.efsa.2015.4318.
- ^Polturak G, Aharoni A (January 2018).""La Vie en Rose": Biosynthesis, Sources, and Applications of Betalain Pigments ".Molecular Plant.11(1): 7–22.doi:10.1016/j.molp.2017.10.008.PMID29081360.
- ^Dean D. Metcalfe, Hugh A. Sampson, Ronald A. Simon:Food Allergy: Adverse Reactions to Foods and Food Additives.4th Ed., Blackwell Publishing, 2009,ISBN978-1-4051-5129-0,p. 416.
- ^Manohar, Cynthya M.; Kundgar, Saurabh D.; Doble, Mukesh (1 July 2017). "Betanin immobilized LDPE as antimicrobial food wrapper".LWT.80:131–135.doi:10.1016/j.lwt.2016.07.020.
- ^Herbach, Kirsten M.; Stintzing, Florian C.; Carle, Reinhold (May 2006)."Betalain Stability and Degradation?Structural and Chromatic Aspects".Journal of Food Science.71(4): R41–R50.doi:10.1111/j.1750-3841.2006.00022.x.ISSN0022-1147.
Further reading
[edit]- Harmer, R.A. (January 1980). "Occurrence, chemistry and application of betanin".Food Chemistry.5(1): 81–90.doi:10.1016/0308-8146(80)90066-7.
