From Wikipedia, the free encyclopedia
Chemical compound
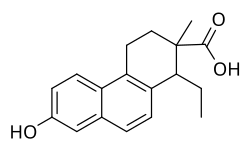
Bisdehydrodoisynolic acid
1-Ethyl-7-hydroxy-2-methyl-1,2,3,4-tetrahydrophenanthrene-2-carboxylic acid
PubChem CID UNII Formula C 18 H 20 O 3 Molar mass −1 3D model (JSmol )
CCC1C2=C(CCC1(C)C(O)=O)C1=CC=C(O)C=C1C=C2
InChI=1/C18H20O3/c1-3-16-15-6-4-11-10-12(19)5-7-13(11)14(15)8-9-18(16,2)17(20)21/h4-7,10,16,19H,3,8-9H2,1-2H3,(H,20,21)
Key:HMYBVYBHZVQZNH-UHFFFAOYNA-N
Bisdehydrodoisynolic acid (BDDA ), as the (Z)-isomer ((Z)-BDDA), is asynthetic ,nonsteroidal estrogen related todoisynolic acid that was never marketed.[ 1] [ 2] [ 3] selective estrogen receptor modulator (SERM).[ 3] [ 4] affinity accompanied by disproportionately high estrogenicpotency in vivo [ 5] metabolites with greater estrogenic activity.[ 4] equilenin ordihydroequilenin withpotassium hydroxide .[ 6] estrone .[ 7] diethylstilbestrol ,can be considered to be open-ring analogues ofestradiol .[ 8] methyl ether of BDDA,doisynoestrol ,is also an estrogen, and in contrast to BDDA, has been marketed.[ 2] [ 9]
^ Banz W, Strader A, Ajuwon K, Ortiz L, James B, Higginbotham DA, Hou Y, Meyers C (May 2007)."The Effects of (+)-Z-bisdehydrodoisynolic Acid on Diabetic Phenotype in Female Obese Zucker Rats" .Experimental Biology .pp. 17–.ISBN 978-0-549-22172-2 [permanent dead link ^a b Johnson WS, Graber RP (1950). "The Stobbe Condensation with 6-Methoxy-2-propionylnaphthalene. A Synthesis of Bisdehydrodoisynolic Acid1".Journal of the American Chemical Society .72 (2): 925–935.doi :10.1021/ja01158a075 .ISSN 0002-7863 . ^a b Blickenstaff RT, Ghosh AC, Wolf GC (22 October 2013).Total Synthesis of Steroids: Organic Chemistry: A Series of Monographs ISBN 978-1-4832-1642-3 ^a b Adler M, Hou Y, Sandrock P, Meyers CY, Winters TA, Banz WJ, Adler S (August 2006)."Derivatives of Z-bisdehydrodoisynolic acid provide a new description of the binding-activity paradox and selective estrogen receptor modulator activity" .Endocrinology .147 (8): 3952–3960.doi :10.1210/en.2006-0316 PMID 16709609 . ^ Banz WJ, Winters TA, Hou Y, Adler S, Meyers CY (December 1998). "Comparative effects of the selective estrogen receptor modulators (-)-, (+)- and (+/-)-Z bisdehydrodoisynolic acids on metabolic and reproductive parameters in male and female rats".Hormone and Metabolic Research .30 (12): 730–736.doi :10.1055/s-2007-978968 .PMID 9930631 . ^ Pincus G, Thimann KV (2 December 2012).The Hormones V1: Physiology, Chemistry and Applications ISBN 978-0-323-14206-9 ^ Journal of Scientific & Industrial Research ^ Morice C ,Wermuth CG (2 May 2011)."Ring transformations" .In Wermuth CG (ed.).The Practice of Medicinal Chemistry .Academic Press. pp. 343-362 (344).ISBN 978-0-08-056877-5 ^ Elks J (14 November 2014)."Doisynoestrol" .The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies .Springer. pp. 465–.ISBN 978-1-4757-2085-3
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g.,testosterone andesters ,methyltestosterone ,metandienone (methandrostenolone) ,nandrolone andesters ,many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g.,anethole ,anol ,dianethole ,dianol ,photoanethole )Chalconoids (e.g.,isoliquiritigenin ,phloretin ,phlorizin (phloridzin) ,wedelolactone )Coumestans (e.g.,coumestrol ,psoralidin )Flavonoids (incl.7,8-DHF ,8-prenylnaringenin ,apigenin ,baicalein ,baicalin ,biochanin A ,calycosin ,catechin ,daidzein ,daidzin ,ECG ,EGCG ,epicatechin ,equol ,formononetin ,glabrene ,glabridin ,genistein ,genistin ,glycitein ,kaempferol ,liquiritigenin ,mirificin ,myricetin ,naringenin ,penduletin ,pinocembrin ,prunetin ,puerarin ,quercetin ,tectoridin ,tectorigenin )Lavender oil Lignans (e.g.,enterodiol ,enterolactone ,nyasol (cis -hinokiresinol) )Metalloestrogens (e.g.,cadmium )Pesticides (e.g.,alternariol ,dieldrin ,endosulfan ,fenarimol ,HPTE ,methiocarb ,methoxychlor ,triclocarban ,triclosan )Phytosteroids (e.g.,digitoxin (digitalis ),diosgenin ,guggulsterone )Phytosterols (e.g.,β-sitosterol ,campesterol ,stigmasterol )Resorcylic acid lactones (e.g.,zearalanone ,α-zearalenol ,β-zearalenol ,zearalenone ,zeranol (α-zearalanol) ,taleranol (teranol, β-zearalanol) )Steroid -like (e.g.,deoxymiroestrol ,miroestrol )Stilbenoids (e.g.,resveratrol ,rhaponticin )Synthetic xenoestrogens (e.g.,alkylphenols ,bisphenols (e.g.,BPA ,BPF ,BPS ),DDT ,parabens ,PBBs ,PHBA ,phthalates ,PCBs )Others (e.g.,agnuside ,rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown