Bisphenol A

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
4,4′-(Propane-2,2-diyl)diphenol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.133 |
| EC Number |
|
| KEGG | |
PubChemCID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2430 |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C15H16O2 | |
| Molar mass | 228.291g·mol−1 |
| Appearance | White solid |
| Odor | Phenolic, medical |
| Density | 1.217 g/cm3[1] |
| Melting point | 155 °C (311 °F; 428 K)[5] |
| Boiling point | 250–252 °C (482–486 °F; 523–525 K)[5]at 13 torrs (0.017 atm) |
| 0.3 g/L (25 °C)[2] | |
| logP | 3.41[3] |
| Vapor pressure | 5×10−6Pa(25 °C)[4] |
| Hazards[6] | |
| GHSlabelling: | |
   
| |
| Danger | |
| H317,H318,H335,H360,H411[6] | |
| P201,P202,P261,P273,P302+P352,P304+P340,P305+P351+P338,P308+P313,P333+P313,P363,P403+P233[6] | |
| NFPA 704(fire diamond) | |
| Flash point | 227 °C (441 °F; 500 K)[6] |
| 510 °C (950 °F; 783 K)[6] | |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Bisphenol A(BPA) is achemical compoundprimarily used in the manufacturing of variousplastics.It is a colourless solid which issolublein most common organicsolvents,but has very poor solubility in water.[2][7]BPA is produced on an industrial scale by thecondensation reactionofphenolandacetone.Global production in 2022 was estimated to be in the region of 10 million tonnes.[8]
BPA's largest single application is as aco-monomerin the production ofpolycarbonates,which accounts for 65–70% of all BPA production.[9][10]The manufacturing ofepoxy resinsandvinyl ester resinsaccount for 25–30% of BPA use.[9][10]The remaining 5% is used as a major component of severalhigh-performance plastics,and as a minor additive inPVC,polyurethane,thermal paper,and several other materials. It is not aplasticizer,[11]although it is often wrongly labelled as such.
The health effects of BPA have been the subject of prolonged public and scientific debate.[12][13][14]BPA is axenoestrogen,exhibiting hormone-like properties that mimic the effects ofestrogenin the body.[15]Although the effect is very weak,[16]the pervasiveness of BPA-containing materials raises concerns, as exposure is effectively lifelong. Many BPA-containing materials are non-obvious but commonly encountered,[17]and include coatings for the inside offood cans,[18]clothing designs,[19]shop receipts,[20]and dental fillings.[21]BPA has been investigated by public health agencies in many countries, as well as by theWorld Health Organization.[12]While normal exposure is below the level currently associated with risk, several jurisdictions have taken steps to reduce exposure on a precautionary basis, in particular by banning BPA from baby bottles. There is some evidence that BPA exposure in infants has decreased as a result of this.[22]BPA-free plastics have also been introduced, which are manufactured using alternative bisphenols such asbisphenol Sandbisphenol F,but there is also controversy around whether these are actually safer.[23][24][25]
History[edit]
Bisphenol A was first reported in 1891 by the RussianchemistAleksandr Dianin.[26]
In 1934, workers atI.G. Farbenindustriereported the coupling of BPA andepichlorohydrin.Over the following decade, coatings and resins derived from similar materials were described by workers at the companies of DeTrey Freres inSwitzerlandand DeVoe and Raynolds in the US. This early work underpinned the development ofepoxy resins,which in turn motivated production of BPA.[27]The utilization of BPA further expanded with discoveries atBayerandGeneral Electriconpolycarbonateplastics.These plastics first appeared in 1958, being produced byMobay,General Electric, and Bayer.[28]
The British biochemist EdwardCharles Doddstested BPA as an artificialestrogenin the early 1930s.[29][30][31]Subsequent work found that it bound toestrogen receptorstens of thousands of times more weakly thanestradiol,the major natural female sex hormone.[32][16]Dodds eventually developed a structurally similar compound,diethylstilbestrol(DES), which was used as a synthetic estrogen drug in women and animals until it was banned due to its risk of causing cancer; the ban on use of DES in humans came in 1971 and in animals, in 1979.[29]BPA was never used as a drug.[29]
Production[edit]
The synthesis of BPA still follows Dianin's general method, with the fundamentals changing little in 130 years. Thecondensationofacetone(hence the suffix 'A' in the name)[33]with twoequivalentsofphenoliscatalyzedby a strong acid, such as concentratedhydrochloric acid,sulfuric acid,or a solid acidresinsuch as thesulfonic acidform ofpolystyrene sulfonate.[34]An excess of phenol is used to ensure full condensation and to limit the formation of byproducts, such asDianin's compound.BPA is fairly cheap to produce, as the synthesis benefits from a highatom economyand large amounts of both starting materials are available from thecumene process.[7]As the onlyby-productis water, it may be considered an industrial example ofgreen chemistry.Global production in 2022 was estimated to be in the region of 10 million tonnes.[8]
Usually, the addition of acetone takes place at thepara positionon both phenols, however minor amounts of the ortho-para (up to 3%) and ortho-ortho isomers are also produced, along with several other minor by‑products.[35]These are not always removed and are known impurities in commercial samples of BPA.[36][35]
Properties[edit]
BPA has a fairly high melting point but can be easily dissolved in a broad range of organic solvents includingtoluene,ethanolandethyl acetate.[37]It may be purified byrecrystallisationfrom acetic acid with water.[38]Crystals form in themonoclinicspace groupP 21/n (where n indicates the glide plane); within this individual molecules of BPA are arraigned with a 91.5°torsion anglebetween the phenol rings.[39][40][41]Spectroscopicdata is available fromAIST.[42]
Uses and applications[edit]

Main uses[edit]
Polycarbonates[edit]
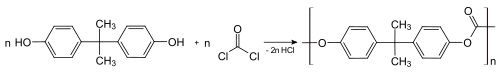
About 65–70% of all bisphenol A is used to makepolycarbonateplastics,[9][10]which can consist of nearly 90% BPA by mass.Polymerisationis achieved by a reaction withphosgene,conducted under biphasic conditions; the hydrochloric acid is scavenged with aqueous base.[43]This process converts the individual molecules of BPA into large polymer chains, effectively trapping them.
Epoxy and vinyl ester resins[edit]
About 25–30% of all BPA is used in the manufacture ofepoxy resinsandvinyl ester resins.[9][10]For epoxy resin, it is first converted to itsdiglycidyl ether(usually abbreviated BADGE or DGEBA).[44][45]This is achieved by a reaction withepichlorohydrinunder basic conditions.
Some of this is further reacted withmethacrylic acidto formbis-GMA,which is used to make vinyl ester resins. Alternatively, and to a much lesser extent, BPA may beethoxylatedand then converted to its diacrylateand dimethacrylatederivatives (bis-EMA, or EBPADMA). These may be incorporated at low levels in vinyl ester resins to change their physical properties[46]and see common use indental compositesandsealants.[47][48]
Minor uses[edit]
The remaining 5% of BPA is used in a wide range of applications, many of which involve plastic.[49]BPA is a main component of severalhigh-performance plastics,the production of these is low compared to other plastics but still equals several thousand tons a year. Comparatively minor amounts of BPA are also used as additives or modifiers in somecommodity plastics.These materials are much more common but their BPA content will be low.
Plastics[edit]
- As a major component
- Polycyanurates can be produced from BPA by way of its dicyanate ester(BADCy).[49]This is formed by a reaction between BPA andcyanogen bromide.[50]Examples includeBT-Epoxy,which is one of a number of resins used in the production ofprinted circuit boards.
- Polyetherimidessuch as Ultem can be produced from BPA via a nitro-displacement of appropriate bisnitroimides.[51][52]Thesethermoplasticpolyimideplastics have exceptional resistance to mechanical, thermal and chemical damage. They are used in medical devices and other high performance instrumentation.
- Polybenzoxazinesmay be produced from a number of biphenols, including BPA.[53][54]
- Polysulfonescan be produced from BPA andbis(4-chlorophenyl) sulfoneforming poly(bisphenol-A sulfone) (PSF). It is used as a high performance alternative to polycarbonate.[49][55]
- Bisphenol-A formaldehyde resins are a subset ofphenol formaldehyde resins.They are used in the production ofhigh-pressure laminates[49]
- As a minor component
- Polyurethanecan incorporate BPA and its derivatives as hard segment chain extenders, particularly inmemory foams.[56][57]
- PVCcan contain BPA and its derivatives through multiple routes. BPA is sometimes used as an antioxidant inphthalates,[58]which are extensively used asplasticizersfor PVC. BPA has also been used as an antioxidant to protect sensitive PVCheat stabilizers.Historically 5–10% by weight of BPA was included in barium-cadmium types, although these have largely been phased out due health concerns surrounding thecadmium.BPA diglycidyl ether (BADGE) is used as an acid scavenger, particularly in PVCdispersions,such as organosols orplastisols,[59][60]which are used as coatings for the inside of food cans, as well as embossed clothes designs produced usingheat transfer vinylorscreen printingmachines.[19]
- BPA is used to form a number offlame retardantsused in plastics.[61]Bromination of BPA formstetrabromobisphenol A(TBBPA), which is mainly used as a reactive component of polymers, meaning that it is incorporated into the polymer backbone. It is used to prepare fire-resistantpolycarbonatesby replacing some bisphenol A. A lower grade of TBBPA is used to prepareepoxy resins,used inprinted circuit boards.TBBPA is also converted to tetrabromobisphenol-A-bis(2,3,-dibromopropyl ether) (TBBPA-BDBPE) which can be used as a flame retardant inpolypropylene.TBBPA-BDBPE is not chemically bonded to the polymer and can leach out into the environment.[62]The use of these compounds is diminishing due to restrictions onbrominated flame retardants.The reaction of BPA withphosphorus oxychlorideandphenolformsbisphenol-A bis(diphenyl phosphate)(BADP), which is used as a liquid flame retarder in some high performancepolymer blendssuch as polycarbonate/ABSmixtures.[63]
Other applications[edit]
- BPA is used as an antioxidant in several fields, particularly inbrake fluids.[64]
- BPA is used as a developing agent inthermal paper(shop receipts).[20]Recycled paper products can also contain BPA,[65]although this can depend strongly on how it is recycled.Deinkingcan remove 95% of BPA,[9]with the pulp produced used to make newsprint, toilet paper and facial tissues. If deinking is not performed then the BPA remains in the fibers, paper recycled this way is usually made intocorrugated fiberboard.[9]
- EthoxylatedBPA finds minor use as a 'levelling agent' in tinelectroplating.
- Several drug candidates have also been developed from bisphenol A, includingralaniten,ralaniten acetate,andEPI-001.
BPA substitutes[edit]
Concerns about the health effects of BPA have led some manufacturers replacing it with other bisphenols, such asbisphenol Sandbisphenol F.These are produced in a similar manner to BPA, by replacing acetone with otherketones,which undergo analogous condensation reactions.[7]Thus, inbisphenol F,the F signifiesformaldehyde. Health concerns have also been raised about these substitutes.[66][24]Alternative polymers, such astritan copolyesterhave been developed to give the same properties as polycarbonate (durable, clear) without using BPA or its analogues.
| Structural formula | Name | CAS | Reactants | |
|---|---|---|---|---|
 |
Bisphenol AF | 1478-61-1 | Phenol | Hexafluoroacetone |
| Bisphenol F | 620-92-8 | Phenol | Formaldehyde | |
 |
Bisphenol S | 80-09-1 | Phenol | Sulfur trioxide |
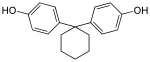 |
Bisphenol Z | 843-55-0 | Phenol | Cyclohexanone |
 |
Tetramethyl bisphenol F | 5384-21-4 | 2,6-xylenol | Formaldehyde |
Human safety[edit]
Exposure[edit]

As a result of the presence of BPA in plastics and other commonplace materials, most people are frequently exposed to trace levels of BPA.[67][68][69]The primary source of human exposure is via food, as epoxy and PVC are used to line the inside of food cans to prevent corrosion of the metal by acidic foodstuffs. Polycarbonate drinks containers are also a source of exposure, although most disposable drinks bottles are actually made ofPET,which contains no BPA. Among the non-food sources, exposures routes include through dust,[10]thermal paper,[20]clothing,[19]dental materials,[70]and medical devices.[17]Although BPA exposure is common it does not accumulate within the body, withtoxicokineticstudies showing thebiological half-lifeof BPA in adult humans to be around two hours.[71][72]The body first converts it into more water-soluble compounds viaglucuronidationorsulfation,which are then removed from the body through the urine. This allows exposure to be easily determined by urine testing, facilitating convenientbiomonitoringof populations.[22][17][73]Food and drink containers made from Bisphenol A-containing plastics do not contaminate the content to cause any increased cancer risk.[74]
Health effects and regulation[edit]
The health effects of BPA have been the subject of prolonged public and scientific debate,[12][13][14]withPubMedlisting more than 18,000 scientific papers as of 2024.[75]Concern is mostly related to itsestrogen-like activity, although it can interact with other receptor systems as anendocrine-disrupting chemical.[76]These interactions are all very weak, but exposure to BPA is effectively lifelong, leading to concern over possible cumulative effects. Studying this sort of long‑term, low‑dose interaction is difficult, and although there have been numerous studies, there are considerable discrepancies in their conclusions regarding the nature of the effects observed as well as the levels at which they occur.[12]A common criticism is that industry-sponsored trials tend to show BPA as being safer than studies performed by academic or government laboratories,[14][77]although this has also been explained in terms of industry studies being better designed.[13][78]
Public health agencies in the EU,[79][80][81]US,[82][83]Canada,[84]Australia[85]and Japan as well as theWHO[12]have all reviewed the health risks of BPA, and found normal exposure to be below the level currently associated with risk. Regardless, due to the scientific uncertainty, many jurisdictions have taken steps to reduce exposure on a precautionary basis. In particular, infants are considered to be at greater risk,[86]leading to bans on the use of BPA inbaby bottlesand related products by the US,[87]Canada,[88]and EU[89]amongst others. Bottle producers have largely switched from polycarbonate topolypropyleneand there is some evidence that BPA exposure in infants has decreased as a result of this.[22]TheEuropean Chemicals Agencyhas added BPA to the Candidate List ofsubstances of very high concern(SVHC), which would make it easier to restrict or ban its use in future.[90][91]In June 2023 after the EFSA reported about the toxicity of BPA the European Union has passed the resolution in early 2024 to ban BPA in all the food contact material including plastic and coated packaging and it said it would also address other bisphenols to avoid replacing with other harmful substances.
BPA exhibits very lowacute toxicity(i.e. from a single large dose) as indicated by itsLD50of 4 g/kg (mouse). Reports indicate that it is a minor skin irritant as well, although less so thanphenol.[7]
Pharmacology[edit]

BPA has been found to interact with a diverse range ofhormone receptors,in both humans and animals.[76]It binds to both of thenuclearestrogen receptors(ERs),ERαandERβ.BPA can both mimic the action ofestrogenandantagoniseestrogen, indicating that it is aselective estrogen receptor modulator(SERM) orpartial agonistof the ER. Although, it is 1000- to 2000-fold less potent thanestradiol,the major female sex hormone in humans. At high concentrations, BPA also binds to and acts as an antagonist of theandrogen receptor(AR). In addition to receptor binding, the compound has been found to affectLeydig cellsteroidogenesis,including affecting17α-hydroxylase/17,20 lyaseandaromataseexpression and interfering withLH receptor-ligand binding.[92]
Bisphenol A's interacts with theestrogen-related receptor γ(ERR-γ). Thisorphan receptor(endogenous ligand unknown) behaves as a constitutive activator of transcription. BPA seems to bind strongly to ERR-γ (dissociation constant= 5.5 nM), but only weakly to the ER.[93]BPA binding to ERR-γ preserves its basal constitutive activity.[93]It can also protect it from deactivation from the SERM4-hydroxytamoxifen(afimoxifene).[93]This may be the mechanism by which BPA acts as axenoestrogen.[93]Different expression of ERR-γ in different parts of the body may account for variations in bisphenol A effects. BPA has also been found to act as anagonistof theGPER(GPR30).[94]
Environmental safety[edit]
Distribution and degradation[edit]
BPA has been detectable in the natural environment since the 1990s and is now widely distributed.[95]It is primarily a river pollutant,[96]but has also been observed in the marine environment,[97]in soils,[98]and lower levels can also be detected in air.[99]The solubility of BPA in water is low (~300 g per ton of water)[2]but this is still sufficient to make it a significant means of distribution into the environment.[98]Many of the largest sources of BPA pollution are water-based, particularly wastewater from industrial facilities using BPA. Paper recyclingcan be a major source of release when this includesthermal paper,[9][100]leachingfrom PVC items may also be a significant source,[96]as can landfillleachate.[101]
In all cases,wastewater treatmentcan be highly effective at removing BPA, giving reductions of 91–98%.[102]Regardless, the remaining 2–9% of BPA will continue through to the environment, with low levels of BPA commonly observed in surface water and sediment in the U.S. and Europe.[103]
Once in the environment BPA is aerobically biodegraded by a wide a variety of organisms.[95][104][105]Itshalf lifein water has been estimated at between 4.5 and 15 days, degradation in the air is faster than this, while soil samples degrade more slowly.[98]BPA in sediment degrades most slowly of all, particularly where this is anaerobic.Abioticdegradation has been reported, but is generally slower than biodegradation. Pathways includephoto-oxidation,or reactions with minerals such asgoethitewhich may be present in soils and sediments.[106]
Environmental effects[edit]
BPA is an environmentalcontaminant of emerging concern.[101]Despite its short half-life and non-bioaccumulatingcharacter, the continuous release of BPA into the environment causes continuous exposure to both plant[107]and animal life. Although many studies have been performed, these often focus on a limited range ofmodel organismsand can use BPA concentrations well beyond environmental levels.[108]As such, the precise effects of BPA on the growth, reproduction, and development of aquatic organism are not fully understood.[108]Regardless, the existing data shows the effects of BPA on wildlife to be generally negative.[109][110]BPA appears able to affect development and reproduction in a wide range of wildlife,[110][111]with certain species being particularly sensitive, such asinvertebratesandamphibians.[109]
See also[edit]
- Structurally related
- 4,4'-Dihydroxybenzophenone- used as a UV stabilizer in cosmetics and plastics
- Dinitrobisphenol A- a proposed metabolite of BPA, which may show increased endocrine disrupting character
- HPTE- a metabolite of the synthetic insecticidemethoxychlor
- Others
- 2,2,4,4-Tetramethyl-1,3-cyclobutanediol- next generation BPA replacement
- 4-tert-Butylphenol- used as a chain-length regulator in the production of polycarbonates and epoxy resins, it has also been studied as a potential endocrine disruptor
References[edit]
- ^Lim CF, Tanski JM (3 August 2007). "Structural Analysis of Bisphenol-A and its Methylene, Sulfur, and Oxygen Bridged Bisphenol Analogs".Journal of Chemical Crystallography.37(9): 587–595.doi:10.1007/s10870-007-9207-8.S2CID97284173.
- ^abcShareef A, Angove MJ, Wells JD, Johnson BB (11 May 2006). "Aqueous Solubilities of Estrone, 17β-Estradiol, 17α-Ethynylestradiol, and Bisphenol A".Journal of Chemical & Engineering Data.51(3): 879–881.doi:10.1021/je050318c.
- ^Robinson BJ, Hui JP, Soo EC, Hellou J (2009). "Estrogenic Compounds in Seawater and Sediment from Halifax Harbour, Nova Scotia, Canada".Environmental Toxicology and Chemistry.28(1): 18–25.doi:10.1897/08-203.1.PMID18702564.S2CID13528747.
- ^"Chemical Fact Sheet – Cas #80057 CASRN 80-05-7".speclab.com.1 April 2012. Archived fromthe originalon 12 February 2012.Retrieved14 June2012.
- ^abMitrofanova SE, Bakirova IN, Zenitova LA, Galimzyanova AR, Nefed'ev ES (September 2009). "Polyurethane varnish materials based on diphenylolpropane".Russian Journal of Applied Chemistry.82(9): 1630–1635.doi:10.1134/S1070427209090225.S2CID98036316.
- ^abcdeSigma-Aldrich Co.,Bisphenol A.
- ^abcdFiege H, Voges HW, Hamamoto T, Umemura S, Iwata T, Miki H, Fujita Y, Buysch HJ, Garbe D, Paulus W (2000). "Phenol Derivatives".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a19_313.ISBN978-3527306732.
- ^abAbraham A, Chakraborty P (June 2020). "A review on sources and health impacts of bisphenol A".Reviews on Environmental Health.35(2): 201–210.doi:10.1515/reveh-2019-0034.PMID31743105.S2CID208186123.
- ^abcdefgEuropean Commission. Joint Research Centre. Institute for Health Consumer Protection (2010).Updated European Union risk assessment report: 4,4'-isopropylidenediphenol (bisphenol-A): environment addendum of February 2008.Publications Office. p. 6.doi:10.2788/40195.ISBN9789279175428.
- ^abcdeVasiljevic T, Harner T (May 2021)."Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels".The Science of the Total Environment.789:148013.Bibcode:2021ScTEn.78948013V.doi:10.1016/j.scitotenv.2021.148013.PMID34323825.
- ^Cadogan DF, Howick CJ (2000). "Plasticizers".Ullmann's Encyclopedia of Industrial Chemistry.doi:10.1002/14356007.a20_439.ISBN3527306730.
- ^abcdeJoint FAO/WHO expert meeting to review toxicological and health aspects of bisphenol A: final report, including report of stakeholder meeting on bisphenol A, 1-5 November 2010, Ottawa, Canada.World Health Organization. 2011.hdl:10665/44624.ISBN978-92-4-156427-4.Retrieved23 March2022.
- ^abcHengstler JG, Foth H, Gebel T, Kramer PJ, Lilienblum W, Schweinfurth H, et al. (April 2011)."Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A".Critical Reviews in Toxicology.41(4): 263–291.doi:10.3109/10408444.2011.558487.PMC3135059.PMID21438738.
- ^abcMyers JP, vom Saal FS, Akingbemi BT, Arizono K, Belcher S, Colborn T, et al. (March 2009)."Why public health agencies cannot depend on good laboratory practices as a criterion for selecting data: the case of bisphenol A".Environmental Health Perspectives.117(3): 309–315.doi:10.1289/ehp.0800173.PMC2661896.PMID19337501.
- ^Egan M (2013). "Sarah A. Vogel. Is It Safe? BPA and the Struggle to Define the Safety of Chemicals".Isis.105(1). Berkeley: University of California Press: 254.doi:10.1086/676809.ISSN0021-1753.
- ^abBlair RM (1 March 2000)."The Estrogen Receptor Relative Binding Affinities of 188 Natural and Xenochemicals: Structural Diversity of Ligands".Toxicological Sciences.54(1): 138–153.doi:10.1093/toxsci/54.1.138.PMID10746941.
- ^abcGeens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, et al. (October 2012). "A review of dietary and non-dietary exposure to bisphenol-A".Food and Chemical Toxicology.50(10): 3725–3740.doi:10.1016/j.fct.2012.07.059.PMID22889897.
- ^Noonan GO, Ackerman LK, Begley TH (July 2011). "Concentration of bisphenol A in highly consumed canned foods on the U.S. market".Journal of Agricultural and Food Chemistry.59(13): 7178–7185.doi:10.1021/jf201076f.PMID21598963.
- ^abcXue J, Liu W, Kannan K (May 2017)."Bisphenols, Benzophenones, and Bisphenol A Diglycidyl Ethers in Textiles and Infant Clothing".Environmental Science & Technology.51(9): 5279–5286.Bibcode:2017EnST...51.5279X.doi:10.1021/acs.est.7b00701.PMID28368574.
- ^abcBjörnsdotter MK, de Boer J, Ballesteros-Gómez A (September 2017)."Bisphenol A and replacements in thermal paper: A review".Chemosphere.182:691–706.Bibcode:2017Chmsp.182..691B.doi:10.1016/j.chemosphere.2017.05.070.hdl:1871.1/0c9480c5-48ce-4955-8d53-39b8b246802f.PMID28528315.
- ^Ahovuo-Saloranta A, Forss H, Walsh T, Nordblad A, Mäkelä M, Worthington HV (July 2017)."Pit and fissure sealants for preventing dental decay in permanent teeth".The Cochrane Database of Systematic Reviews.2017(7): CD001830.doi:10.1002/14651858.CD001830.pub5.PMC6483295.PMID28759120.
- ^abcHuang RP, Liu ZH, Yin H, Dang Z, Wu PX, Zhu NW, Lin Z (June 2018). "Bisphenol A concentrations in human urine, human intakes across six continents, and annual trends of average intakes in adult and child populations worldwide: A thorough literature review".The Science of the Total Environment.626:971–981.Bibcode:2018ScTEn.626..971H.doi:10.1016/j.scitotenv.2018.01.144.PMID29898562.S2CID49194096.
- ^Thoene M, Dzika E, Gonkowski S, Wojtkiewicz J (February 2020)."Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A Literature Review".Nutrients.12(2): 532.doi:10.3390/nu12020532.PMC7071457.PMID32092919.
- ^abChen D, Kannan K, Tan H, Zheng Z, Feng YL, Wu Y, Widelka M (7 June 2016). "Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity—A Review".Environmental Science & Technology.50(11): 5438–5453.Bibcode:2016EnST...50.5438C.doi:10.1021/acs.est.5b05387.PMID27143250.
- ^Eladak S, Grisin T, Moison D, Guerquin MJ, N'Tumba-Byn T, Pozzi-Gaudin S, Benachi A, Livera G, Rouiller-Fabre V, Habert R (2015)."A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound".Fertility and Sterility.103(1): 11–21.doi:10.1016/j.fertnstert.2014.11.005.PMID25475787.
- ^See:
- А. Дианина (1891)"О продуктахъ конденсацiи кетоновъ съ фенолами"(On condensation products of ketones with phenols),Журнал Русского физико-химического общества(Journal of the Russian Physical Chemistry Society),23:488-517, 523–546, 601–611; see especially pages 491-493 ( "Диметилдифенолметань" (dimethyldiphenolmethane)).
- Reprinted in condensed form in: A. Dianin (1892)"Condensationsproducte aus Ketonen und Phenolen"(Condensation products of ketones and phenols),Berichte der Deutschen chemischen Gesellschaft zu Berlin,25,part 3: 334-337.doi:10.1002/cber.18920250333
- ^Pham HQ, Marks MJ (2012). "Epoxy Resins".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a09_547.pub2.ISBN978-3527306732.
- ^Serini V (2000). "Polycarbonates".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a21_207.ISBN978-3527306732.
- ^abcVogel SA (November 2009)."The politics of plastics: the making and unmaking of bisphenol a" safety "".American Journal of Public Health.99(Suppl 3): S559–S566.doi:10.2105/AJPH.2008.159228.PMC2774166.PMID19890158.
- ^Dodds EC, Lawson W (1936)."Synthetic Œstrogenic Agents without the Phenanthrene Nucleus".Nature.137(3476): 996.Bibcode:1936Natur.137..996D.doi:10.1038/137996a0.S2CID4171635.
- ^Dodds EC, Lawson W (1938)."Molecular Structure in Relation to Oestrogenic Activity. Compounds without a Phenanthrene Nucleus".Proceedings of the Royal Society of London B: Biological Sciences.125(839): 222–232.Bibcode:1938RSPSB.125..222D.doi:10.1098/rspb.1938.0023.
- ^Kwon JH, Katz LE, Liljestrand HM (October 2007). "Modeling binding equilibrium in a competitive estrogen receptor binding assay".Chemosphere.69(7): 1025–1031.Bibcode:2007Chmsp..69.1025K.doi:10.1016/j.chemosphere.2007.04.047.PMID17559906.
- ^Uglea CV, Negulescu II (1991).Synthesis and Characterization of Oligomers.CRC Press.p. 103.ISBN978-0-8493-4954-6.
- ^De Angelis A, Ingallina P, Perego C (March 2004). "Solid Acid Catalysts for Industrial Condensations of Ketones and Aldehydes with Aromatics".Industrial & Engineering Chemistry Research.43(5): 1169–1178.doi:10.1021/ie030429+.
- ^abTerasaki M, Nomachi M, Edmonds JS, Morita M (May 2004). "Impurities in industrial grade 4,4'-isopropylidene diphenol (bisphenol A): possible implications for estrogenic activity".Chemosphere.55(6): 927–931.Bibcode:2004Chmsp..55..927T.doi:10.1016/j.chemosphere.2003.11.063.PMID15041297.
- ^Pahigian JM, Zuo Y (September 2018)."Occurrence, endocrine-related bioeffects and fate of bisphenol A chemical degradation intermediates and impurities: A review".Chemosphere.207:469–480.Bibcode:2018Chmsp.207..469P.doi:10.1016/j.chemosphere.2018.05.117.PMID29807346.S2CID44172964.
- ^Haynes WM (2017).CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data(2016-2017, 97th ed.). Boca Raton, Florida. pp. 3–56.ISBN9781498754293.
{{cite book}}:CS1 maint: location missing publisher (link) - ^Perrin DD, Armarego WL (1988).Purification of laboratory chemicals.Butterworth-Heinemann. p. 208.ISBN9780080347141.
- ^"2,2-bis(4-Hydroxyphenyl)propane".www.ccdc.cam.ac.uk.The Cambridge Crystallographic Data Centre.Retrieved29 June2022.
- ^Okada K (July 1996). "X-ray crystal structure analyses and atomic charges of color former and developer. I. Color developers".Journal of Molecular Structure.380(3): 223–233.Bibcode:1996JMoSt.380..223O.doi:10.1016/0022-2860(95)09168-8.
- ^Wolak JE, Knutson J, Martin JD, Boyle P, Sargent AL, White JL (1 December 2003). "Dynamic Disorder and Conformer Exchange in the Crystalline Monomer of Polycarbonate".The Journal of Physical Chemistry B.107(48): 13293–13299.doi:10.1021/jp036527q.
- ^"4,4'-isopropylidenediphenol".sdbs.db.aist.go.jp.Spectral Database for Organic Compounds (SDBS).Retrieved29 June2022.
- ^Serini V (2000). "Polycarbonates".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a21_207.ISBN978-3527306732.
- ^Ng F, Couture G, Philippe C, Boutevin B, Caillol S (January 2017)."Bio-Based Aromatic Epoxy Monomers for Thermoset Materials".Molecules.22(1): 149.doi:10.3390/molecules22010149.PMC6155700.PMID28106795.
- ^Kroschwitz JI (1998).Kirk-Othmer Encyclopedia of Chemical Technology.Vol. 5 (5 ed.). Wiley. p. 8.ISBN978-0-471-52695-7.
- ^Gonçalves F, Kawano Y, Pfeifer C, Stansbury JW, Braga RR (August 2009). "Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites".European Journal of Oral Sciences.117(4): 442–446.doi:10.1111/j.1600-0722.2009.00636.x.PMID19627357.
- ^Sideridou I, Tserki V, Papanastasiou G (April 2002). "Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins".Biomaterials.23(8): 1819–1829.doi:10.1016/S0142-9612(01)00308-8.PMID11950052.
- ^Sideridou ID, Achilias DS (July 2005). "Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC".Journal of Biomedical Materials Research Part B: Applied Biomaterials.74B(1): 617–626.doi:10.1002/jbm.b.30252.PMID15889433.
- ^abcdGeens T, Goeyens L, Covaci A (September 2011). "Are potential sources for human exposure to bisphenol-A overlooked?".International Journal of Hygiene and Environmental Health.214(5): 339–347.Bibcode:2011IJHEH.214..339G.doi:10.1016/j.ijheh.2011.04.005.PMID21570349.
- ^Hamerton I (1994).Chemistry and technology of cyanate ester resins(1st ed.). London: Blackie Academic & Professional.ISBN978-0-7514-0044-1.
- ^Takekoshi T, Kochanowski JE, Manello JS, Webber MJ (June 1985). "Polyetherimides. I. Preparation of dianhydrides containing aromatic ether groups".Journal of Polymer Science: Polymer Chemistry Edition.23(6): 1759–1769.Bibcode:1985JPoSA..23.1759T.doi:10.1002/pol.1985.170230616.
- ^Lau KS (2014). "10 - High-Performance Polyimides and High Temperature Resistant Polymers".Handbook of thermoset plastics(3rd ed.). San Diego: William Andrew. pp. 319–323.ISBN978-1-4557-3107-7.
- ^Vijayakumar CT, Shamim Rishwana S, Surender R, David Mathan N, Vinayagamoorthi S, Alam S (2 January 2014)."Structurally diverse benzoxazines: synthesis, polymerization, and thermal stability".Designed Monomers and Polymers.17(1): 47–57.doi:10.1080/15685551.2013.797216.S2CID94255723.
- ^Ghosh NN, Kiskan B, Yagci Y (November 2007). "Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties".Progress in Polymer Science.32(11): 1344–1391.doi:10.1016/j.progpolymsci.2007.07.002.
- ^"Kirk-Othmer Encyclopedia of Chemical Technology". 4 December 2000.doi:10.1002/0471238961.0118151323080920.a01.
{{cite journal}}:Cite journal requires|journal=(help) - ^Laza JM, Veloso A, Vilas JL (10 January 2021). "Tailoring new bisphenol a ethoxylated shape memory polyurethanes".Journal of Applied Polymer Science.138(2): 49660.doi:10.1002/app.49660.S2CID224955435.
- ^Król P (2008).Linear polyurethanes: synthesis methods, chemical structures, properties and applications.Leiden: VSP. pp. 11–14.ISBN9789004161245.
- ^"European Union Summary Risk Assessment Report - Bis (2-ethylhexyl) Phthalate (DEHP)".Joint Research Centre (JRC) Publications Repository.European Commission. 16 July 2008.ISSN1018-5593.Retrieved24 November2021.

- ^Shah AC, Poledna DJ (September 2003). "Review of PVC dispersion and blending resin products".Journal of Vinyl and Additive Technology.9(3): 146–154.doi:10.1002/vnl.10076.S2CID98016356.
- ^Shah AC, Poledna DJ (September 2002). "Review of specialty PVC resins".Journal of Vinyl and Additive Technology.8(3): 214–221.doi:10.1002/vnl.10365.S2CID97146596.
- ^Dagani MJ, Barda HJ, Benya TJ, Sanders DC. "Bromine Compounds".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a04_405.ISBN978-3527306732.
- ^Gauthier LT, Laurich B, Hebert CE, Drake C, Letcher RJ (20 August 2019). "Tetrabromobisphenol-A-Bis(dibromopropyl ether) Flame Retardant in Eggs, Regurgitates, and Feces of Herring Gulls from Multiple North American Great Lakes Locations".Environmental Science & Technology.53(16): 9564–9571.Bibcode:2019EnST...53.9564G.doi:10.1021/acs.est.9b02472.PMID31364365.S2CID198998658.
- ^Pawlowski KH, Schartel B (November 2007). "Flame retardancy mechanisms of triphenyl phosphate, resorcinol bis(diphenyl phosphate) and bisphenol A bis(diphenyl phosphate) in polycarbonate/acrylonitrile–butadiene–styrene blends".Polymer International.56(11): 1404–1414.doi:10.1002/pi.2290.
- ^Lamprea K, Bressy A, Mirande-Bret C, Caupos E, Gromaire MC (August 2018)."Alkylphenol and bisphenol A contamination of urban runoff: an evaluation of the emission potentials of various construction materials and automotive supplies"(PDF).Environmental Science and Pollution Research International.25(22): 21887–21900.Bibcode:2018ESPR...2521887L.doi:10.1007/s11356-018-2272-z.PMID29796891.S2CID44140721.
- ^Liao C, Kannan K (November 2011). "Widespread occurrence of bisphenol A in paper and paper products: implications for human exposure".Environmental Science & Technology.45(21): 9372–9379.Bibcode:2011EnST...45.9372L.doi:10.1021/es202507f.PMID21939283.
- ^Rochester JR, Bolden AL (July 2015)."Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes".Environmental Health Perspectives.123(7): 643–650.doi:10.1289/ehp.1408989.PMC4492270.PMID25775505.
- ^Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL (January 2008)."Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004".Environmental Health Perspectives.116(1): 39–44.doi:10.1289/ehp.10753.PMC2199288.PMID18197297.
- ^Thoene M, Rytel L, Nowicka N, Wojtkiewicz J (May 2018)."The state of bisphenol research in the lesser developed countries of the EU: a mini-review".Toxicology Research.7(3): 371–380.doi:10.1039/c8tx00064f.PMC6062254.PMID30090587.
- ^Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV (August 2007). "Human exposure to bisphenol A (BPA)".Reproductive Toxicology.24(2): 139–177.doi:10.1016/j.reprotox.2007.07.010.PMID17825522.
- ^Van Landuyt K, Nawrot T, Geebelen B, De Munck J, Snauwaert J, Yoshihara K, Scheers H, Godderis L, Hoet P, Van Meerbeek B (August 2011). "How much do resin-based dental materials release? A meta-analytical approach".Dental Materials.27(8): 723–747.doi:10.1016/j.dental.2011.05.001.PMID21664675.
- ^Tsukioka T, Terasawa JI, Sato S, Hatayama Y, Makino T, Nakazawa H (2004)."Development of Analytical Method for Determining Trace Amounts of BPA in Urine Samples and Estimation of Exposure to BPA".Journal of Environmental Chemistry.14(1): 57–63.doi:10.5985/jec.14.57.
- ^Shin BS, Kim CH, Jun YS, Kim DH, Lee BM, Yoon CH, et al. (December 2004). "Physiologically based pharmacokinetics of bisphenol A".Journal of Toxicology and Environmental Health. Part A.67(23–24): 1971–1985.Bibcode:2004JTEHA..67.1971S.doi:10.1080/15287390490514615.PMID15513896.S2CID24467830.
- ^Bousoumah R, Leso V, Iavicoli I, Huuskonen P, Viegas S, Porras SP, Santonen T, Frery N, Robert A, Ndaw S (August 2021)."Biomonitoring of occupational exposure to bisphenol A, bisphenol S and bisphenol F: A systematic review".Science of the Total Environment.783:146905.Bibcode:2021ScTEn.78346905B.doi:10.1016/j.scitotenv.2021.146905.hdl:10400.21/13242.PMID33865140.S2CID233290894.
- ^"Does using plastic bottles and containers cause cancer?".Cancer Research UK.23 December 2021.
- ^"bisphenol a - Search Results - PubMed".PubMed.Retrieved26 January2024.
- ^abMacKay H, Abizaid A (May 2018). "A plurality of molecular targets: The receptor ecosystem for bisphenol-A (BPA)".Hormones and Behavior.101:59–67.doi:10.1016/j.yhbeh.2017.11.001.PMID29104009.S2CID23088708.
- ^vom Saal FS, Hughes C (2005)."An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment".Environ. Health Perspect.113(8): 926–33.doi:10.1289/ehp.7713.PMC1280330.PMID16079060.
- ^Teeguarden JG, Hanson-Drury S (December 2013). "A systematic review of Bisphenol A" low dose "studies in the context of human exposure: A case for establishing standards for reporting" low-dose "effects of chemicals".Food and Chemical Toxicology.62:935–948.doi:10.1016/j.fct.2013.07.007.PMID23867546.
- ^"Bisphenol A - ECHA".echa.europa.eu.Archived fromthe originalon 8 June 2022.Retrieved28 March2022.
- ^European Food Safety Authority (2015).EFSA explains the Safety of Bisphenol A: scientific opinion on bisphenol A (2015).European Food Safety Authority.doi:10.2805/075460.ISBN9789291996421.
- ^"Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs".EFSA Journal.13(1): 3978. 21 January 2015.doi:10.2903/j.efsa.2015.3978.hdl:2164/12119.
- ^OCSPP US EPA (21 September 2015)."Risk Management for Bisphenol A (BPA)".www.epa.gov.Retrieved28 March2022.
- ^CLARITY-BPA Research Program (October 2021).NTP Research Report on the Consortium Linking Academic and Regulatory Insights on Bisphenol A Toxicity (CLARITY-BPA): A Compendium of Published Findings.p. 18.doi:10.22427/NTP-RR-18.PMID34910417.S2CID240266384.
- ^Health Canada (16 April 2013)."Bisphenol A (BPA)".www.canada.ca (Health Canada).Government of Canada.Retrieved28 March2022.
- ^"Bisphenol A (BPA)".Food Standards Australia New Zealand (FSANZ).Department of Health (Australia).Retrieved28 March2022.
- ^Aschberger K, Castello P, Hoekstra E (2010).Bisphenol A and baby bottles: challenges and perspectives.The Publications Office of the European Union.doi:10.2788/97553.ISBN9789279158698.
- ^"Indirect Food Additives: Polymers".Federal Register.U.S. Government Publishing Office.77FR41899
- ^Legislative Services Branch (1 July 2020)."Consolidated federal laws of canada, Canada Consumer Product Safety Act".laws-lois.justice.gc.ca.
- ^"EUR-Lex - 32011L0008 - EN - EUR-Lex".EUR-Lex.European Union.
COMMISSION DIRECTIVE 2011/8/EU of 28 January 2011 amending Directive 2002/72/EC as regards the restriction of use of Bisphenol A in plastic infant feeding bottles
- ^"MSC unanimously agrees that Bisphenol A is an endocrine disruptor - All news - ECHA".echa.europa.eu.European Chemicals Agency (ECHA).Retrieved19 June2017.
- ^Fisher D (12 July 2019)."EU court confirms BPA as substance of 'very high concern'".Environmental Health News.Retrieved21 July2020.
- ^Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP (1 February 2004)."Inhibition of Testicular Steroidogenesis by the Xenoestrogen Bisphenol A Is Associated with Reduced Pituitary Luteinizing Hormone Secretion and Decreased Steroidogenic Enzyme Gene Expression in Rat Leydig Cells".Endocrinology.145(2): 592–603.doi:10.1210/en.2003-1174.PMID14605012.
- ^abcdMatsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, et al. (October 2007). "Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma".Journal of Biochemistry.142(4): 517–524.doi:10.1093/jb/mvm158.PMID17761695.
- ^Prossnitz ER, Barton M (May 2014)."Estrogen biology: new insights into GPER function and clinical opportunities".Molecular and Cellular Endocrinology.389(1–2): 71–83.doi:10.1016/j.mce.2014.02.002.PMC4040308.PMID24530924.
- ^abStaples CA, Dome PB, Klecka GM, Oblock ST, Harris LR (April 1998). "A review of the environmental fate, effects, and exposures of bisphenol A".Chemosphere.36(10): 2149–2173.Bibcode:1998Chmsp..36.2149S.doi:10.1016/S0045-6535(97)10133-3.PMID9566294.
- ^abCorrales J, Kristofco LA, Steele WB, Yates BS, Breed CS, Williams ES, Brooks BW (29 July 2015)."Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation".Dose-Response.13(3): 1559325815598308.doi:10.1177/1559325815598308.PMC4674187.PMID26674671.
- ^Ozhan K, Kocaman E (February 2019). "Temporal and Spatial Distributions of Bisphenol A in Marine and Freshwaters in Turkey".Archives of Environmental Contamination and Toxicology.76(2): 246–254.Bibcode:2019ArECT..76..246O.doi:10.1007/s00244-018-00594-6.PMID30610254.S2CID58536418.
- ^abcCousins I, Staples C, Kleĉka G, Mackay D (July 2002). "A Multimedia Assessment of the Environmental Fate of Bisphenol A".Human and Ecological Risk Assessment.8(5): 1107–1135.Bibcode:2002HERA....8.1107C.doi:10.1080/1080-700291905846.S2CID43509780.
- ^Vasiljevic T, Harner T (October 2021)."Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels".Science of the Total Environment.789:148013.Bibcode:2021ScTEn.789n8013V.doi:10.1016/j.scitotenv.2021.148013.PMID34323825.
- ^Fürhacker M, Scharf S, Weber H (September 2000). "Bisphenol A: emissions from point sources".Chemosphere.41(5): 751–756.Bibcode:2000Chmsp..41..751F.doi:10.1016/S0045-6535(99)00466-X.PMID10834378.
- ^abQi C, Huang J, Wang B, Deng S, Wang Y, Yu G (2018)."Contaminants of emerging concern in landfill leachate in China: A review".Emerging Contaminants.4(1): 1–10.doi:10.1016/j.emcon.2018.06.001.
- ^Drewes JE, Hemming J, Ladenburger SJ, Schauer J, Sonzogni W (2005). "An assessment of endocrine disrupting activity changes during wastewater treatment through the use of bioassays and chemical measurements".Water Environment Research.77(1): 12–23.Bibcode:2005WaEnR..77...12D.doi:10.2175/106143005x41573.PMID15765931.S2CID12283834.
- ^Klecka GM, Staples CA, Clark KE, Van der Hoeven N, Thomas DE, Hentges SG (August 2009). "Exposure analysis of bisphenol A in surface water systems in North America and Europe".Environmental Science & Technology.43(16): 6145–50.Bibcode:2009EnST...43.6145K.doi:10.1021/es900598e.PMID19746705.
- ^Kang J, Katayama Y, Kondo F (16 January 2006). "Biodegradation or metabolism of bisphenol A: From microorganisms to mammals".Toxicology.217(2–3): 81–90.doi:10.1016/j.tox.2005.10.001.PMID16288945.
- ^Zhang C, Li Y, Wang C, Niu L, Cai W (2 January 2016). "Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: A review".Critical Reviews in Environmental Science and Technology.46(1): 1–59.Bibcode:2016CREST..46....1Z.doi:10.1080/10643389.2015.1061881.S2CID94353391.
- ^Im J, Löffler FE (16 August 2016). "Fate of Bisphenol A in Terrestrial and Aquatic Environments".Environmental Science & Technology.50(16): 8403–8416.Bibcode:2016EnST...50.8403I.doi:10.1021/acs.est.6b00877.OSTI1470902.PMID27401879.
- ^Xiao C, Wang L, Zhou Q, Huang X (February 2020). "Hazards of bisphenol A (BPA) exposure: A systematic review of plant toxicology studies".Journal of Hazardous Materials.384:121488.Bibcode:2020JHzM..38421488X.doi:10.1016/j.jhazmat.2019.121488.PMID31699483.S2CID207939269.
- ^abRubin AM, Seebacher F (July 2022). "Bisphenols impact hormone levels in animals: A meta-analysis".Science of the Total Environment.828:154533.Bibcode:2022ScTEn.82854533R.doi:10.1016/j.scitotenv.2022.154533.PMID35288143.S2CID247423338.
- ^abWu NC, Seebacher F (July 2020). "Effect of the plastic pollutant bisphenol A on the biology of aquatic organisms: A meta-analysis".Global Change Biology.26(7): 3821–3833.Bibcode:2020GCBio..26.3821W.doi:10.1111/gcb.15127.PMID32436328.S2CID218765595.
- ^abOehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, Wollenberger L, Santos EM, Paull GC, Van Look KJ, Tyler CR (2009)."A critical analysis of the biological impacts of plasticizers on wildlife".Philosophical Transactions of the Royal Society B: Biological Sciences.364(1526): 2047–62.doi:10.1098/rstb.2008.0242.PMC2873012.PMID19528055.
- ^Wu NC, Rubin AM, Seebacher F (26 January 2022)."Endocrine disruption from plastic pollution and warming interact to increase the energetic cost of growth in a fish".Proceedings of the Royal Society B: Biological Sciences.289(1967).doi:10.1098/rspb.2021.2077.ISSN0962-8452.PMC8790379.PMID35078359.