C2 domain
| C2 domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
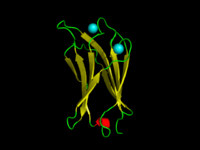 The C2-domain ofC.absonumα-toxin (PDB 1OLP). β-strands are shown in yellow, α-helices in red, loops in green. Co-ordinatedcalciumions are in cyan. | |||||||||
| Identifiers | |||||||||
| Symbol | C2 | ||||||||
| Pfam | PF00168 | ||||||||
| InterPro | IPR000008 | ||||||||
| SMART | C2 | ||||||||
| PROSITE | PDOC00380 | ||||||||
| SCOP2 | 1qas/SCOPe/SUPFAM | ||||||||
| OPM superfamily | 45 | ||||||||
| OPM protein | 1ugk | ||||||||
| CDD | cd00030 | ||||||||
| |||||||||
| Phosphoinositide 3-kinase C2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
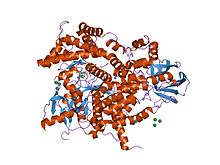 Structure of phosphoinositide 3-kinase.[1] | |||||||||||
| Identifiers | |||||||||||
| Symbol | PI3K_C2 | ||||||||||
| Pfam | PF00792 | ||||||||||
| InterPro | IPR002420 | ||||||||||
| SMART | PI3K_C2 | ||||||||||
| PROSITE | PDOC50004 | ||||||||||
| SCOP2 | 1e8x/SCOPe/SUPFAM | ||||||||||
| CDD | cd08380 | ||||||||||
| |||||||||||
AC2 domainis aproteinstructural domaininvolved in targeting proteins tocell membranes.The typical version (PKC-C2) has abeta-sandwichcomposed of 8β-strandsthat co-ordinates two or threecalciumions, which bind in a cavity formed by the first and final loops of the domain, on the membrane binding face. Many other C2 domain families don't have calcium binding activity.[2][3]
Coupling with other domains
[edit]C2 domains are frequently found coupled toenzymaticdomains; for example, the C2 domain inPTEN,brings thephosphatasedomain into contact with the plasma membrane, where it can dephosphorylate its substrate,phosphatidylinositol (3,4,5)-trisphosphate (PIP3),without removing it from the membrane - which would be energetically very costly. PTEN consists of two domains, aprotein tyrosine phosphatasedomain and a C2 domain. This domain pair constitutes a superdomain, a heritable unit that is found in various proteins in fungi, plants and animals.[4]In addition,phosphatidylinositol 3-kinase(PI3-kinase), an enzyme thatphosphorylatesphosphoinositideson the 3-hydroxyl groupof theinositolring, also uses a C2 domain to bind to the membrane (e.g. 1e8w PDB entry).
Evolution
[edit]The C2 domain is currently only known from eukaryotes and the prokaryoteClostridium perfringenswhere it is part of thealpha-toxin.[5]Over 17 distinct clades of C2 domains have been identified.[2][3]Most C2 families can be traced back tobasaleukaryotic species indicating an early diversification before the last eukaryotic common ancestor (LECA). Only the PKC-C2 domain family contains conserved calcium-binding residues, suggesting the typical calcium-dependent membrane interaction is a derived feature limited in PKC-C2 domains.[2]
Calcium and lipid selectivity
[edit]C2 domains are unique among membrane targeting domains in that they show wide range of lipid selectivity for the major components of cell membranes, including phosphatidylserine and phosphatidylcholine. This C2 domain is about 116 amino-acid residues and is located between the two copies of the C1 domain in Protein Kinase C (that bind phorbol esters and diacylglycerol) (seePDOC00379) and the protein kinase catalytic domain (seePDOC00100). Regions with significant homology[6]to the C2-domain have been found in many proteins. The C2 domain is thought to be involved in calcium-dependent phospholipid binding[7]and in membrane targeting processes such as subcellular localisation. Although most C2 domains interact with the membrane (phospholipids) in a Ca2+-dependent manner, some C2 domains can interact with the membrane without binding to Ca2+.Similarly, C2 domains have been evolved to have different specificities for lipids. Many C2 domains such assynaptotagminC2A, bind to anionic phospholipids (PS or PIP2 containing phospholipids). However, other C2 domains such as cPLA2-α C2 domain bind to zwitterionic lipids (e.g. PC). This diversity and selectivity in Ca2+and lipid binding suggest that C2 domains are evolved to have different functions.[8]
3D structure
[edit]The domain forms an eight-stranded beta sandwich constructed around a conserved 4-stranded motif, designated a C2 key.[9]Calcium binds in a cup-shaped depression formed by the N- and C-terminal loops of the C2-key motif. Structural analyses of several C2 domains have shown them to consist of similar ternary structures in which three Ca2+-binding loops are located at the end of an 8 stranded antiparallel beta sandwich.
Human proteins containing C2 domain
[edit]ABR;BAIAP3;BCR;C2CD2;C2CD3;CADPS;CADPS2; CAPN5;CAPN6;CC2D1A;CC2D1B;CPNE1;CPNE2;CPNE3;CPNE4; CPNE5;CPNE6;CPNE7;CPNE8;CPNE9;DAB2IP;DOC2A;DOC2B; DYSF;ESYT1;ESYT3;FAM62A;FAM62B;FAM62C;FER1L3;FER1L5; HECW1;HECW2;ITCH;ITSN1;ITSN2; MCTP1;MCTP2;MTAC2D1;NEDD4;NEDD4L;NEDL1;OTOF; PCLO;PIK3C2A;PIK3C2B;PIK3C2G;PLA2G4A;PLA2G4B;PLA2G4D;PLA2G4E; PLA2G4F;PLCB1;PLCB2;PLCB3;PLCB4;PLCD1;PLCD3;PLCD4; PLCE1;PLCG1;PLCG2;PLCH1;PLCH2;PLCL1;PLCL2;PLCZ1; PRF1;PRKCA;PRKCB1;PRKCE;PRKCG;PRKCH;RAB11FIP1;RAB11FIP2; RAB11FIP5;RASA1;RASA2;RASA3;RASA4;RASAL1;RASAL2;RGS3; RIMS1;RIMS2;RIMS3;RIMS4;RPGRIP1;RPGRIP1L;RPH3A;SGA72M; SMURF1;SMURF2;SYNGAP1;SYT1;SYT10;SYT11;SYT12;SYT13; SYT14;SYT14L;SYT15;SYT16;SYT17;SYT2;SYT3;SYT4; SYT5;SYT6;SYT7;SYT8;SYT9;SYTL1;SYTL2;SYTL3; SYTL4;SYTL5;TOLLIP;UNC13A;UNC13B;UNC13C;UNC13D;WWC2; WWP1;WWP2; PTEN
References
[edit]- ^Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL (October 2000)."Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine".Molecular Cell.6(4): 909–19.doi:10.1016/S1097-2765(05)00089-4.PMID11090628.
- ^abcZhang D, Aravind L (December 2010)."Identification of novel families and classification of the C2 domain superfamily elucidate the origin and evolution of membrane targeting activities in eukaryotes".Gene.469(1–2): 18–30.doi:10.1016/j.gene.2010.08.006.PMC2965036.PMID20713135.
- ^abZhang D, Aravind L (October 2012)."Novel transglutaminase-like peptidase and C2 domains elucidate the structure, biogenesis and evolution of the ciliary compartment".Cell Cycle.11(20): 3861–75.doi:10.4161/cc.22068.PMC3495828.PMID22983010.
- ^Haynie DT, Xue B (May 2015)."Superdomains in the protein structure hierarchy: The case of PTP-C2".Protein Science.24(5): 874–82.doi:10.1002/pro.2664.PMC4420535.PMID25694109.
- ^Naylor, Claire E.; Eaton, Julian T.; Howells, Angela; Justin, Neil; Moss, David S.; Titball, Richard W.; Basak, Ajit K. (August 1998). "Structure of the key toxin in gas gangrene".Nature Structural & Molecular Biology.5(8): 738–746.doi:10.1038/1447.ISSN1545-9993.PMID9699639.S2CID21000585.
- ^Brose N, Hofmann K, Hata Y, Südhof TC (October 1995)."Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins".The Journal of Biological Chemistry.270(42): 25273–80.doi:10.1074/jbc.270.42.25273.PMID7559667.
- ^Davletov BA, Südhof TC (December 1993)."A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding".The Journal of Biological Chemistry.268(35): 26386–90.doi:10.1016/S0021-9258(19)74326-9.PMID8253763.
- ^Nalefski EA, Wisner MA, Chen JZ, Sprang SR, Fukuda M, Mikoshiba K, Falke JJ (March 2001)."C2 domains from different Ca2+ signaling pathways display functional and mechanistic diversity".Biochemistry.40(10): 3089–100.doi:10.1021/bi001968a.PMC3862187.PMID11258923.
- ^Sutton RB, Davletov BA, Berghuis AM, Südhof TC, Sprang SR (March 1995)."Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold".Cell.80(6): 929–38.doi:10.1016/0092-8674(95)90296-1.PMID7697723.S2CID18981505.
External links
[edit]- Phosphoinositide 3-kinase C2 family in Pfam
- UMich Orientation of Proteins in Membranesfamilies/superfamily-47- Orientations of C2 domains in membranes (OPM)
