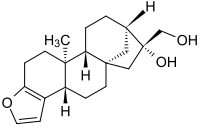
Cafestol
| |
| Names | |
|---|---|
| IUPAC name
3,18-(Epoxymetheno)-19-nor-5β,8α,9β,10α,13β,16β-kaur-3-ene-16α,17-diol
| |
| Systematic IUPAC name
(3bS,5aS,7R,8R,10aR,10bS)-7-(Hydroxymethyl)-10b-methyl-3b,4,5,6,7,8,9,10,10a,10b,11,12-dodecahydro-5a,8-methanocyclohepta[5,6]naphtho[2,1-b]furan-7-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C20H28O3 | |
| Molar mass | 316.441g·mol−1 |
| Melting point | 158 to 162 °C (316 to 324 °F; 431 to 435 K) |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Cafestolis aditerpenoidmoleculepresent incoffeebeans. It is one of the compounds that may be responsible for proposed biological and pharmacological effects of coffee.[1]
Sources
[edit]A typical bean ofCoffea arabicacontains about 0.4% to 0.7% cafestol by weight.[2]Cafestol is present in highest quantity in unfiltered coffee drinks such asFrench presscoffee,Turkish coffeeorGreek coffee.Inpaper-filtered coffeedrinks such asdrip brewedcoffee, it is present in only negligible amounts, as the paper filter in drip filtered coffee retains the diterpenes.[3]
Research into biological activity
[edit]Coffee consumption has been associated with a number of effects on health and cafestol has been proposed to produce these through a number of biological actions.[4]Studies have shown that regular consumption of boiled coffee increasesserum cholesterolwhereas filtered coffee does not.[5]Cafestol may act as anagonistligandfor thenuclear receptorfarnesoid X receptorandpregnane X receptor,blocking cholesterolhomeostasis.Thus cafestol can increase cholesterol synthesis.[6]
Cafestol has also shownanticarcinogenicproperties in rats.[7]
Cafestol also has neuroprotective effects in aDrosophilafruit fly model ofParkinson's disease.[8][9]
See also
[edit]References
[edit]- ^Ludwig, I. A.; Clifford, M. N.; Lean, M. E.; Ashihara, H.; Crozier, A. (August 2014). "Coffee: biochemistry and potential impact on health".Food & Function.5(8): 1695–1717.doi:10.1039/c4fo00042k.PMID24671262.S2CID29389074.
- ^Kitzberger, C.; Scholz, M.; Benassi, M. (2014). "Bioactive compounds content in roasted coffee from traditional and modernCoffea arabicacultivars grown under the same edapho-climatic conditions ".Food Research International.61:61–66.doi:10.1016/j.foodres.2014.04.031.
- ^Zhang, C.; Linforth, R.; Fisk, I. D. (2012)."Cafestol extraction yield from different coffee brew mechanisms".Food Research International.49:27–31.doi:10.1016/j.foodres.2012.06.032.
- ^Higdon, J. V.; Frei, B. (2006). "Coffee and health: a review of recent human research".Critical Reviews in Food Science and Nutrition.46(2): 101–123.doi:10.1080/10408390500400009.PMID16507475.
- ^Urgert, R.; Katan, M. B. (1997)."The cholesterol-raising factor from coffee beans".Annual Review of Nutrition.17:305–324.doi:10.1146/annurev.nutr.17.1.305.PMC1295997.PMID9240930.
- ^Ricketts, M. L.; Boekschoten, M. V.; Kreeft, A. J.; Hooiveld, G. J.; Moen, C. J.; Müller, M.; Frants, R. R.; Kasanmoentalib, S.; Post, S. M.; Princen, H. M.; Porter, J. G.; Katan, M. B.; Hofker, M. H.; Moore, D. D. (2007)."The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors".Molecular Endocrinology.21(7): 1603–1616.doi:10.1210/me.2007-0133.PMID17456796.
- ^National Toxicology Program(October 1999)."Cafestol (CASRN 469-83-0) and Kahweol (CASRN 6894-43-5) — Review of Toxicological Literature"(PDF).Archived fromthe original(PDF)on November 1, 2004.
- ^Trinh, K.; Andrews, L.; Krause, J.; Hanak, T.; Lee, D.; Gelb, M.; Pallanck, L. (April 2010)."Decaffeinated coffee and nicotine-free tobacco provide neuroprotection inDrosophilamodels of Parkinson's disease through an NRF2-dependent mechanism ".The Journal of Neuroscience.30(16): 5525–5532.doi:10.1523/JNEUROSCI.4777-09.2010.PMC3842467.PMID20410106.
- ^Callaway, E. (April 23, 2010)."Parkinson's protection without caffeine or nicotine".New Scientist.
