Phenol
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Phenol[1] | |||
| Systematic IUPAC name
Benzenol | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.003.303 | ||
| KEGG | |||
PubChemCID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2821 (solution) 2312 (molten) 1671 (solid) | ||
CompTox Dashboard(EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H6O | |||
| Molar mass | 94.113g/mol | ||
| Appearance | Transparent crystalline solid | ||
| Odor | Sweet and tarry | ||
| Density | 1.07g/cm3 | ||
| Melting point | 40.5 °C (104.9 °F; 313.6 K) | ||
| Boiling point | 181.7 °C (359.1 °F; 454.8 K) | ||
| 8.3g/100 mL (20°C) | |||
| logP | 1.48[2] | ||
| Vapor pressure | 0.4mmHg (20°C)[3] | ||
| Acidity(pKa) |
| ||
| Conjugate base | Phenoxide | ||
| UV-vis(λmax) | 270.75nm[5] | ||
| 1.224D | |||
| Pharmacology | |||
| C05BB05(WHO)D08AE03(WHO),N01BX03(WHO),R02AA19(WHO) | |||
| Hazards | |||
| GHSlabelling: | |||
   [6] [6]
| |||
| Danger | |||
| H301,H311,H314,H331,H341,H373[6] | |||
| P261,P280,P301+P310,P305+P351+P338,P310[6] | |||
| NFPA 704(fire diamond) | |||
| Flash point | 79 °C (174 °F; 352 K) | ||
| Explosive limits | 1.8–8.6%[3] | ||
| Lethal doseor concentration (LD, LC): | |||
LD50(median dose)
|
| ||
LDLo(lowest published)
|
| ||
LC50(median concentration)
|
| ||
| NIOSH(US health exposure limits): | |||
PEL(Permissible)
|
TWA 5ppm (19mg/m3) [skin][3] | ||
REL(Recommended)
|
| ||
IDLH(Immediate danger)
|
250ppm[3] | ||
| Safety data sheet(SDS) | [1] | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |||
Phenol(also known ascarbolic acid,phenolic acid,orbenzenol) is anaromaticorganic compoundwith the molecularformulaC6H5OH.[5]It is a whitecrystallinesolidthat isvolatile.The molecule consists of aphenyl group(−C6H5) bonded to ahydroxy group(−OH). Mildlyacidic,it requires careful handling because it can causechemical burns.[5]
Phenol was first extracted fromcoal tar,but today is produced on a large scale (about 7 million tonnes a year) frompetroleum-derived feedstocks. It is an important industrialcommodityas aprecursorto many materials and useful compounds.[8]It is primarily used to synthesizeplasticsand related materials. Phenol and its chemicalderivativesare essential for production ofpolycarbonates,epoxies,explosives,Bakelite,nylon,detergents,herbicidessuch asphenoxy herbicides,and numerouspharmaceutical drugs.[9]
Properties
[edit]Phenol is an organic compound appreciablysolublein water, with about 84.2 g dissolving in 1000 ml (0.895M). Homogeneous mixtures of phenol and water at phenol to water mass ratios of ~2.6 and higher are possible. The sodium salt of phenol,sodium phenoxide,is far more water-soluble. It is a combustible solid (NFPA rating = 2). When heated, phenol produces flammable vapors that are explosive at concentrations of 3 to 10% in air. Carbon dioxide or dry chemical extinguishers should be used to fight phenol fires.[5]
Acidity
[edit]Phenol is a weak acid (pH 6.6). In aqueous solution in the pH range ca. 8 - 12 it is in equilibrium with thephenolateanionC6H5O−(also calledphenoxideorcarbolate):[10]

Phenol is more acidic than aliphatic alcohols. Its enhanced acidity is attributed toresonance stabilizationofphenolateanion. In this way, the negative charge on oxygen is delocalized on to theortho and paracarbon atoms through the pi system.[11]An alternative explanation involves the sigma framework, postulating that the dominant effect is theinductionfrom the more electronegativesp2hybridised carbons;the comparatively more powerful inductive withdrawal of electron density that is provided by the sp2system compared to an sp3system allows for great stabilization of the oxyanion. In support of the second explanation, thepKaof theenolofacetonein water is 10.9, making it only slightly less acidic than phenol (pKa10.0).[5]Thus, the greater number of resonance structures available to phenoxide compared to acetone enolate seems to contribute little to its stabilization. However, the situation changes when solvation effects are excluded.
Hydrogen bonding
[edit]Incarbon tetrachlorideand in alkane solvents, phenolhydrogen bondswith a wide range of Lewis bases such aspyridine,diethyl ether,anddiethyl sulfide.The enthalpies of adduct formation and the−OHIR frequency shifts accompanying adduct formation have been compiled.[12]Phenol is classified as ahard acid.[13][14]
Tautomerism
[edit]
Phenol exhibitsketo-enol tautomerismwith its unstable keto tautomer cyclohexadienone, but the effect is nearly negligible. The equilibrium constant for enolisation is approximately 10−13,which means only one in every ten trillion molecules is in the keto form at any moment.[15]The small amount of stabilisation gained by exchanging a C=C bond for a C=O bond is more than offset by the large destabilisation resulting from the loss of aromaticity. Phenol therefore exists essentially entirely in the enol form.[16]4, 4' Substituted cyclohexadienone can undergo adienone–phenol rearrangementin acid conditions and form stable 3,4‐disubstituted phenol.[17]
For substituted phenols, several factors can favor the keto tautomer: (a) additional hydroxy groups (seeresorcinol) (b) annulation as in the formation ofnaphthols,and (c) deprotonation to give the phenolate.[18]
Phenoxides areenolatesstabilised byaromaticity.Under normal circumstances, phenoxide is more reactive at the oxygen position, but the oxygen position is a "hard" nucleophile whereas the alpha-carbon positions tend to be "soft".[19]
Reactions
[edit]
Phenol is highly reactive towardelectrophilic aromatic substitution.The enhanced nucleophilicity is attributed to donationpi electrondensity from O into the ring. Many groups can be attached to the ring, viahalogenation,acylation,sulfonation,and related processes.
Phenol is so strongly activated that bromination and chlorination lead readily to polysubstitution.[20]The reaction affords 2- and 4-substituted derivatives. The regiochemistry of halogenation changes in strongly acidic solutions wherePhOH2]+predominates. Phenol reacts with dilute nitric acid at room temperature to give a mixture of 2-nitrophenol and 4-nitrophenol while with concentrated nitric acid, additional nitro groups are introduced, e.g. to give2,4,6-trinitrophenol.Friedel Crafts alkylationsof phenol and its derivatives often proceed without catalysts. Alkylating agents include alkyl halides, alkenes, and ketones. Thus,adamantyl-1-bromide,dicyclopentadiene), andcyclohexanonesgive respectively 4-adamantylphenol, a bis(2-hydroxyphenyl) derivative, and a 4-cyclohexylphenols.Alcoholsandhydroperoxidesalkylate phenols in the presence ofsolid acidcatalysts(e.g. certainzeolite).Cresolsand cumyl phenols can be produced in that way.[21]
Aqueous solutions of phenol are weakly acidic and turn blue litmus slightly to red. Phenol is neutralized bysodium hydroxideforming sodium phenate or phenolate, but being weaker thancarbonic acid,it cannot be neutralized bysodium bicarbonateorsodium carbonateto liberatecarbon dioxide.
- C6H5OH + NaOH → C6H5ONa + H2O
When a mixture of phenol andbenzoyl chlorideare shaken in presence of dilutesodium hydroxidesolution,phenyl benzoateis formed. This is an example of theSchotten–Baumann reaction:
- C6H5COCl + HOC6H5→ C6H5CO2C6H5+ HCl
Phenol is reduced tobenzenewhen it is distilled withzincdust or when its vapour is passed over granules of zinc at 400 °C:[22]
- C6H5OH + Zn → C6H6+ ZnO
When phenol is treated withdiazomethanein the presence ofboron trifluoride(BF3),anisoleis obtained as the main product and nitrogen gas as a byproduct.
- C6H5OH + CH2N2→ C6H5OCH3+ N2
Phenol and its derivatives react with iron(III) chloride to give intensely colored solutions containing phenoxide complexes.
Production
[edit]Because of phenol's commercial importance, many methods have been developed for its production, but the cumene process is the dominant technology.
Cumene process
[edit]
Accounting for 95% of production (2003) is thecumene process,also calledHock process.It involves the partialoxidationofcumene(isopropylbenzene) via theHock rearrangement:[8]Compared to most other processes, the cumene process uses mild conditions and inexpensive raw materials. For the process to be economical, both phenol and the acetone by-product must be in demand.[23][24]In 2010, worldwide demand for acetone was approximately 6.7 million tonnes, 83 percent of which was satisfied with acetone produced by the cumene process.
A route analogous to the cumene process begins withcyclohexylbenzene.It isoxidizedto ahydroperoxide,akin to the production ofcumene hydroperoxide.Via the Hock rearrangement, cyclohexylbenzene hydroperoxide cleaves to give phenol andcyclohexanone.Cyclohexanone is an important precursor to somenylons.[25]
Oxidation of benzene, toluene, cyclohexylbenzene
[edit]The direct oxidation ofbenzene(C6H6) to phenol is theoretically possible and of great interest, but it has not been commercialized:
- C6H6+ O → C6H5OH
Nitrous oxideis a potentially "green" oxidant that is a more potent oxidant than O2.Routes for the generation of nitrous oxide however remain uncompetitive.[26][23][25]
Anelectrosynthesisemployingalternating currentgives phenol from benzene.[27]
The oxidation oftoluene,as developed byDow Chemical,involves copper-catalyzed reaction of molten sodium benzoate with air:
- C6H5CH3+ 2 O2→ C6H5OH + CO2+ H2O
The reaction is proposed to proceed via formation of benzyoylsalicylate.[8]
Autoxidationof cyclohexylbenzene give thehydroperoxide.Decomposition of this hydroperoxide affordscyclohexanoneand phenol.[8]
Older methods
[edit]Early methods relied on extraction of phenol from coal derivatives or the hydrolysis of benzene derivatives.
Hydrolysis of benzenesulfonic acid
[edit]The original commercial route was developed byBayerandMonsantoin the early 1900s, based on discoveries by Wurtz and Kekule. The method involves the reaction of strong base withbenzenesulfonic acid,proceeding by the reaction of hydroxide withsodium benzenesulfonateto give sodium phenoxide. Acidification of the latter gives phenol. The net conversion is:[28]
- C6H5SO3H + 2 NaOH → C6H5OH + Na2SO3+ H2O
Hydrolysis of chlorobenzene
[edit]Chlorobenzenecan be hydrolyzed to phenol using base (Dow process) or steam (Raschig–Hooker process):[24][25][29]
- C6H5Cl + NaOH → C6H5OH + NaCl
- C6H5Cl + H2O → C6H5OH + HCl
These methods suffer from the cost of the chlorobenzene and the need to dispose of the chloride byproduct.
Coal pyrolysis
[edit]Phenol is also a recoverable byproduct ofcoalpyrolysis.[29]In theLummus process,the oxidation of toluene tobenzoic acidis conducted separately.
Miscellaneous methods
[edit]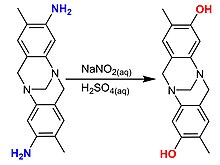
Phenyldiazoniumsalts hydrolyze to phenol. The method is of no commercial interest since the precursor is expensive.[30]
Salicylic aciddecarboxylates to phenol.[31]
Uses
[edit]The major uses of phenol, consuming two thirds of its production, involve its conversion to precursors for plastics.Condensationwith acetone givesbisphenol-A,a key precursor topolycarbonatesandepoxideresins. Condensation of phenol, alkylphenols[citation needed],or diphenols[citation needed]withformaldehydegivesphenolic resins,a famous example of which isBakelite.Partialhydrogenationof phenol givescyclohexanone,[32]a precursor tonylon.Nonionicdetergentsare produced by alkylation of phenol to give thealkylphenols,e.g.,nonylphenol,which are then subjected toethoxylation.[8]
Phenol is also a versatile precursor to a large collection of drugs, most notablyaspirinbut also manyherbicidesandpharmaceutical drugs.Phenol is a component inliquid–liquidphenol–chloroform extractiontechnique used inmolecular biologyfor obtainingnucleic acidsfrom tissues or cell culture samples. Depending on the pH of the solution eitherDNAorRNAcan be extracted.
Phenol is so inexpensive that it also attracts many small-scale uses. It is a component of industrialpaint strippersused in the aviation industry for the removal of epoxy, polyurethane and other chemically resistant coatings.[33]Due to safety concerns, phenol is banned from use in cosmetic products in theEuropean Union[34][35]andCanada.[36][37]
Medical
[edit]Phenol was widely used as anantiseptic,and it is used in the production ofcarbolic soap.Concentrated phenol liquids are used for permanent treatment of ingrown toe and finger nails, a procedure known as a chemicalmatrixectomy.The procedure was first described by Otto Boll in 1945. Since that time phenol has become the chemical of choice for chemical matrixectomies performed by podiatrists.
Concentrated liquid phenol can be used topically as a local anesthetic forotologyprocedures, such asmyringotomyandtympanotomytube placement, as an alternative to general anesthesia or other local anesthetics. It also has hemostatic and antiseptic qualities that make it ideal for this use. Phenol spray, usually at 1.4% phenol as an active ingredient, is used medically to treat sore throat.[38]It is the active ingredient in some oral analgesics such asChlorasepticspray,TCPandCarmex.[39]
History
[edit]
Phenol was discovered in 1834 byFriedlieb Ferdinand Runge,who extracted it (in impure form) fromcoal tar.[41]Runge called phenol "Karbolsäure" (coal-oil-acid, carbolic acid). Coal tar remained the primary source until the development of thepetrochemical industry.French chemistAuguste Laurentextracted phenol in its pure form, as a derivative of benzene, in 1841.[42]In 1836, Auguste Laurent coined the name "phène" for benzene;[43]this is the root of the word "phenol" and "phenyl".In 1843, French chemistCharles Gerhardtcoined the name "phénol".[44]
Theantisepticproperties of phenol were used by SirJoseph Listerin his pioneering technique of antiseptic surgery. Lister decided that the wounds had to be thoroughly cleaned. He then covered the wounds with a piece of rag or lint[45]covered in phenol. The skin irritation caused by continual exposure to phenol eventually led to the introduction of aseptic (germ-free) techniques in surgery. Lister's work was inspired by the works and experiments of his contemporaryLouis Pasteurin sterilizing various biological media. He theorized that if germs could be killed or prevented, no infection would occur. Lister reasoned that a chemical could be used to destroy the micro-organisms that cause infection.[46]
Meanwhile, inCarlisle,England, officials were experimenting withsewage treatmentusing carbolic acid to reduce the smell of sewagecesspools.Having heard of these developments, and having previously experimented with other chemicals for antiseptic purposes without much success, Lister decided to try carbolic acid as a wound antiseptic. He had his first chance on August 12, 1865, when he received a patient: an eleven-year-old boy with a tibia bone fracture which pierced the skin of his lower leg. Ordinarily, amputation would be the only solution. However, Lister decided to try carbolic acid. After setting the bone and supporting the leg with splints, he soaked clean cotton towels in undiluted carbolic acid and applied them to the wound, covered with a layer of tin foil, leaving them for four days. When he checked the wound, Lister was pleasantly surprised to find no signs of infection, just redness near the edges of the wound from mild burning by the carbolic acid. Reapplying fresh bandages with diluted carbolic acid, the boy was able to walk home after about six weeks of treatment.[47]
By 16 March 1867, when the first results of Lister's work were published in the Lancet, he had treated a total of eleven patients using his new antiseptic method. Of those, only one had died, and that was through a complication that was nothing to do with Lister's wound-dressing technique. Now, for the first time, patients with compound fractures were likely to leave the hospital with all their limbs intact
- — Richard Hollingham,Blood and Guts: A History of Surgery,p. 62[47]
Before antiseptic operations were introduced at the hospital, there were sixteen deaths in thirty-five surgical cases. Almost one in every two patients died. After antiseptic surgery was introduced in the summer of 1865, there were only six deaths in forty cases. The mortality rate had dropped from almost 50 per cent to around 15 per cent. It was a remarkable achievement
- — Richard Hollingham,Blood and Guts: A History of Surgery,p. 63[48]
Phenol was the main ingredient of the "carbolic smoke ball," an ineffective device marketed in London in the 19th century as protection against influenza and other ailments, and the subject of the famous law caseCarlill v Carbolic Smoke Ball Company.
Second World War
[edit]The toxic effect of phenol on the central nervous system causes sudden collapse and loss of consciousness in both humans and animals; a state of cramping precedes these symptoms because of the motor activity controlled by the central nervous system.[49]Injections of phenol were used as a means of individual execution byNazi Germanyduring theSecond World War.[50]It was originally used by the Nazis in 1939 as part of the mass-murder of disabled people underAktion T4.[51]The Germans learned that extermination of smaller groups was more economical by injection of each victim with phenol. Phenol injections were given to thousands of people.Maximilian Kolbewas also murdered with a phenol injection after surviving two weeks of dehydration and starvation inAuschwitzwhen he volunteered to die in place ofa stranger.Approximately one gram is sufficient to cause death.[52]
Occurrences
[edit]Phenol is a normal metabolic product, excreted in quantities up to 40 mg/L in human urine.[49]Thetemporal glandsecretion of maleelephantsshowed the presence of phenol and4-methylphenolduringmusth.[53][54]It is also one of the chemical compounds found incastoreum.This compound is ingested from the plants the beaver eats.[55]
Phenol is a measurable component in the aroma and taste of the distinctiveIslay scotch whisky,[56]generally ~30ppm,but it can be over 160ppm in the maltedbarleyused to producewhisky.[57]This amount is different from and presumably higher than the amount in the distillate.[56]
Biodegradation
[edit]Cryptanaerobacter phenolicusis a bacterium species that producesbenzoatefrom phenol via4-hydroxybenzoate.[58]Rhodococcus phenolicusis a bacterium species able to degrade phenol as sole carbon source.[59]
Toxicity
[edit]Phenol and its vapors are corrosive to the eyes, the skin, and the respiratory tract.[60]Its corrosive effect on skin and mucous membranes is due to a protein-degenerating effect.[49]Repeated or prolonged skin contact with phenol may causedermatitis,or even second and third-degree burns.[61]Inhalation of phenol vapor may cause lungedema.[60]The substance may cause harmful effects on the central nervous system and heart, resulting indysrhythmia,seizures,andcoma.[62]Thekidneysmay be affected as well. Long-term or repeated exposure of the substance may have harmful effects on theliverandkidneys.[63]There is no evidence that phenol causescancerin humans.[64]Besides itshydrophobiceffects, another mechanism for the toxicity of phenol may be the formation ofphenoxylradicals.[65]
Since phenol is absorbed through the skin relatively quickly, systemic poisoning can occur in addition to the local caustic burns.[49]Resorptive poisoning by a large quantity of phenol can occur even with only a small area of skin, rapidly leading to paralysis of the central nervous system and a severe drop in body temperature. TheLD50for oral toxicity is less than 500 mg/kg for dogs, rabbits, or mice; the minimum lethal human dose was cited as 140 mg/kg.[49]The Agency for Toxic Substances and Disease Registry (ATSDR), U.S. Department of Health and Human Services states the fatal dose for ingestion of phenol is from 1 to 32 g.[66]
Chemical burnsfromskinexposures can be decontaminated by washing withpolyethylene glycol,[67]isopropyl alcohol,[68]or perhaps even copious amounts of water.[69]Removal of contaminated clothing is required, as well as immediatehospitaltreatment for large splashes. This is particularly important if the phenol is mixed withchloroform(a commonly used mixture in molecular biology forDNAandRNApurification).[citation needed]Phenol is also a reproductive toxin causing increased risk of miscarriage and low birth weight indicating retarded development in utero.[5]
Phenols
[edit]The wordphenolis also used to refer to any compound that contains a six-memberedaromaticring, bonded directly to ahydroxyl group(-OH). Thus, phenols are a class oforganic compoundsof which the phenol discussed in this article is the simplest member.
See also
[edit]References
[edit]- ^"Front Matter".Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book).Cambridge:The Royal Society of Chemistry.2014. p. 690.doi:10.1039/9781849733069-FP001(inactive 2024-04-14).ISBN978-0-85404-182-4.
Only one name is retained, phenol, for C6H5-OH, both as a preferred name and for general nomenclature.
{{cite book}}:CS1 maint: DOI inactive as of April 2024 (link) - ^"Phenol_msds".
- ^abcdeNIOSH Pocket Guide to Chemical Hazards."#0493".National Institute for Occupational Safety and Health(NIOSH).
- ^Kütt, Agnes; Movchun, Valeria; Rodima, Toomas; et al. (2008). "Pentakis(trifluoromethyl)phenyl, a Sterically Crowded and Electron-withdrawing Group: Synthesis and Acidity of Pentakis(trifluoromethyl)benzene, -toluene, -phenol, and -aniline".The Journal of Organic Chemistry.73(7): 2607–20.doi:10.1021/jo702513w.PMID18324831.
- ^abcdef"Phenol".PubChem, US National Library of Medicine. 10 June 2023.Retrieved12 June2023.
- ^abcSigma-Aldrich Co.,Phenol.Retrieved on 2022-02-15.
- ^abc"Phenol".Immediately Dangerous to Life or Health Concentrations (IDLH).National Institute for Occupational Safety and Health(NIOSH).
- ^abcdeWeber, Manfred; Weber, Markus; Kleine-Boymann, Michael (2004). "Phenol".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a19_299.pub3.ISBN978-3527306732.
- ^Zvi Rappoport, ed. (2003).The Chemistry of Phenols.PATAI'S Chemistry of Functional Groups. John Wiley & Sons.doi:10.1002/0470857277.ISBN9780470857274.
- ^Smith, Michael B.;March, Jerry(2007),Advanced Organic Chemistry: Reactions, Mechanisms, and Structure(6th ed.), New York: Wiley-Interscience,ISBN978-0-471-72091-1
- ^Organic Chemistry2nd Ed. John McMurryISBN0-534-07968-7
- ^Drago, R S. Physical Methods For Chemists, (Saunders College Publishing 1992), ISBN 0-03-075176-4
- ^Laurence, C. and Gal, J-F. Lewis Basicity and Affinity Scales, Data and Measurement, (Wiley 2010) pp 50-51 ISBN 978-0-470-74957-9
- ^Cramer, R. E.; Bopp, T. T. (1977). "Graphical display of the enthalpies of adduct formation for Lewis acids and bases".Journal of Chemical Education.54:612–613.doi:10.1021/ed054p612.The plots shown in this paper used older parameters. Improved E&C parameters are listed inECW model.
- ^Capponi, Marco; Gut, Ivo G.; Hellrung, Bruno; Persy, Gaby; Wirz, Jakob (1999). "Ketonization equilibria of phenol in aqueous solution".Can. J. Chem.77(5–6): 605–613.doi:10.1139/cjc-77-5-6-605.
- ^Clayden, Jonathan;Greeves, Nick;Warren, Stuart;Wothers, Peter(2001).Organic Chemistry(1st ed.). Oxford University Press. p. 531.ISBN978-0-19-850346-0.
- ^Arnold, Richard T.; Buckley, Jay S. (1 May 1949). "The Dienone-Phenol Rearrangement. II. Rearrangement of 1-Keto-4-methyl-4-phenyl-1,4-dihydronaphthalene".J. Am. Chem. Soc.71(5): 1781.doi:10.1021/ja01173a071.
- ^Sergei M. Lukyanov, Alla V. Koblik (2003). "Tautomeric Equilibria and Rearrangements Involving Phenols". In Zvi Rappoport (ed.).The Chemistry of Phenols.PATAI'S Chemistry of Functional Groups. John Wiley & Sons. pp. 713–838.doi:10.1002/0470857277.ch11.ISBN0471497371.
- ^David Y. Curtin & Allan R. Stein (1966)."2,6,6-Trimethyl-2,4-Cyclohexadione".Organic Syntheses.46:115.doi:10.15227/orgsyn.046.0115.Archived fromthe originalon 2011-06-05.Retrieved2010-03-31.
- ^Muller F, Caillard L (2011). "Chlorophenols".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a07_001.pub2.ISBN978-3527306732.
- ^V. Prakash Reddy. G. K. Surya Prakash (2003). "Electrophilic reactions of phenols". In Zvi Rappoport (ed.).The Chemistry of Phenols.PATAI'S Chemistry of Functional Groups. John Wiley & Sons. pp. 605–660.doi:10.1002/0470857277.ch9.ISBN0471497371.
- ^Roscoe, Henry (1891).A treatise on chemistry, Volume 3, Part 3.London: Macmillan & Co. p. 23.
- ^ab"Phenol -- The essential chemical industry online".2017-01-11.Retrieved2018-01-02.
- ^ab"Direct Routes to Phenol".Archived fromthe originalon 2007-04-09.Retrieved2007-04-09.
- ^abcPlotkin, Jeffrey S. (2016-03-21)."What's New in Phenol Production?".American Chemical Society. Archived fromthe originalon 2019-10-27.Retrieved2018-01-02.
- ^Parmon, V. N.; Panov, G. I.; Uriarte, A.; Noskov, A. S. (2005). "Nitrous oxide in oxidation chemistry and catalysis application and production".Catalysis Today.100(2005): 115–131.doi:10.1016/j.cattod.2004.12.012.
- ^Lee, Byungik; Naito, Hiroto; Nagao, Masahiro; Hibino, Takashi (9 July 2012). "Alternating-Current Electrolysis for the Production of Phenol from Benzene".Angewandte Chemie International Edition.51(28): 6961–6965.doi:10.1002/anie.201202159.PMID22684819.
- ^Wittcoff, H.A., Reuben, B.G. Industrial Organic Chemicals in Perspective. Part One: Raw Materials and Manufacture. Wiley-Interscience, New York. 1980.
- ^abFranck, H.-G., Stadelhofer, J.W. Industrial Aromatic Chemistry. Springer-Verlag, New York. 1988. pp. 148-155.
- ^abKazem-Rostami, Masoud (2017). "Amine to phenol conversion".Synlett.28(13): 1641–1645.doi:10.1055/s-0036-1588180.S2CID99294625.
- ^Kaeding, Warren W. (1 September 1964). "Oxidation of Aromatic Acids. IV. Decarboxylation of Salicylic Acids".The Journal of Organic Chemistry.29(9): 2556–2559.doi:10.1021/jo01032a016.
- ^Musser, Michael T. "Cyclohexanol and Cyclohexanone".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a08_217.pub2.ISBN978-3527306732.
- ^"CH207 Aircraft paintstripper, phenolic, acid"(PDF).Callington. 14 October 2009. Archived fromthe original(PDF)on 23 September 2015.Retrieved25 August2015.
- ^"Prohibited substances in cosmetic product (Annex II, #1175, Phenol) - European Commission".ec.europa.eu.Retrieved2018-07-06.
- ^"CosIng - Cosmetics - GROWTH - European Commission".ec.europa.eu.Retrieved2018-07-06.
- ^Canada, Health (2004-06-18)."Cosmetic Ingredient Hotlist - Canada.ca".www.canada.ca.Retrieved2018-07-06.
- ^Canada, Health (2004-06-18)."Cosmetic Ingredient Hotlist: Prohibited and Restricted Ingredients - Canada.ca".www.canada.ca.Retrieved2018-07-06.
- ^"Phenol spray".drugs.com.
- ^"How Does Our Lip Balm Work".Carmex.Archived fromthe originalon 18 February 2015.Retrieved18 February2015.
- ^"182.095 | Collections Online".collections.thackraymuseum.co.uk.Retrieved2024-05-30.
- ^F. F. Runge (1834)"Ueber einige Produkte der Steinkohlendestillation"(On some products of coal distillation),Annalen der Physik und Chemie,31:65-78. On page 69 of volume 31, Runge names phenol "Karbolsäure" (coal-oil-acid, carbolic acid). Runge characterizes phenol in: F. F. Runge (1834)"Ueber einige Produkte der Steinkohlendestillation,"Annalen der Physik und Chemie,31:308-328.
- ^Auguste Laurent (1841)"Mémoire sur le phényle et ses dérivés"(Memoir on benzene and its derivatives),Annales de Chimie et de Physique,series 3,3:195-228. On page 198, Laurent names phenol "hydrate de phényle" and "l'acide phénique".
- ^Auguste Laurent (1836) "Sur la chlorophénise et les acides chlorophénisique et chlorophénèsique,"Annales de Chemie et de Physique,vol. 63, pp. 27–45, seep. 44:Je donne le nom de phène au radical fondamental des acides précédens (φαινω, j'éclaire), puisque la benzine se trouve dans le gaz de l'éclairage. (I give the name of "phène" (φαινω, I illuminate) to the fundamental radical of the preceding acid, because benzene is found in illuminating gas.)
- ^Gerhardt, Charles (1843)"Recherches sur la salicine,"Annales de Chimie et de Physique,series 3,7:215-229. Gerhardt coins the name "phénol" on page 221.
- ^Lister, Joseph (1867)."Antiseptic Principle Of The Practice Of Surgery".
- ^Hollingham, Richard (2008).Blood and Guts: A History of Surgery.BBC Books - Random House. p. 61.ISBN9781407024530.
- ^abHollingham, Richard (2008).Blood and Guts: A History of Surgery.BBC Books - Random House. p. 62.ISBN9781407024530.
- ^Hollingham, Richard (2008).Blood and Guts: A History of Surgery.BBC Books - Randomhouse. p. 63.ISBN9781407024530.
- ^abcde"Phenol".Ullmann's Encyclopedia of Industrial Chemistry.Vol. 25. Wiley-VCH. 2003. pp. 589–604.
- ^The Experimentsby Peter Tyson. NOVA
- ^The Nazi DoctorsArchived2017-10-22 at theWayback Machine,Chapter 14, Killing with Syringes: Phenol Injections. By Dr. Robert Jay Lifton
- ^"Killing through phenol injection".Auschwitz: Final Station Extermination.Linz, Austria: Johannes Kepler University. Archived fromthe originalon 2006-11-12.
- ^Rasmussen, L.E.L; Perrin, Thomas E (1999). "Physiological Correlates of Musth".Physiology & Behavior.67(4): 539–49.doi:10.1016/S0031-9384(99)00114-6.PMID10549891.S2CID21368454.
- ^Musth in elephants. Deepa Ananth, Zoo's print journal, 15(5), pages 259-262 (article)
- ^The Beaver: Its Life and Impact. Dietland Muller-Schwarze, 2003, page 43 (book at google books)
- ^ab"Peat, Phenol and PPM, by Dr P. Brossard"(PDF).Retrieved2008-05-27.
- ^"Bruichladdich".Bruichladdich.BDCL. Archived fromthe originalon 21 April 2016.Retrieved8 August2015.
- ^Juteau, P.; Côté, V; Duckett, MF; Beaudet, R; Lépine, F; Villemur, R; Bisaillon, JG (2005)."Cryptanaerobacter phenolicusgen. nov., sp. nov., an anaerobe that transforms phenol into benzoate via 4-hydroxybenzoate ".International Journal of Systematic and Evolutionary Microbiology.55(Pt 1): 245–50.doi:10.1099/ijs.0.02914-0.PMID15653882.
- ^Rehfuss, Marc; Urban, James (2005). "Rhodococcus phenolicussp. nov., a novel bioprocessor isolated actinomycete with the ability to degrade chlorobenzene, dichlorobenzene and phenol as sole carbon sources ".Systematic and Applied Microbiology.28(8): 695–701.doi:10.1016/j.syapm.2005.05.011.PMID16261859.
- ^abBudavari, S, ed. (1996).The Merck Index: An Encyclopedia of Chemical, Drugs, and Biologicals.Whitehouse Station, NJ:Merck.
- ^Lin TM, Lee SS, Lai CS, Lin SD (June 2006). "Phenol burn".Burns: Journal of the International Society for Burn Injuries.32(4): 517–21.doi:10.1016/j.burns.2005.12.016.PMID16621299.
- ^Warner, MA; Harper, JV (1985)."Cardiac dysrhythmias associated with chemical peeling with phenol".Anesthesiology.62(3): 366–7.doi:10.1097/00000542-198503000-00030.PMID2579602.
- ^World Health Organization/International Labour Organization: International Chemical Safety Cards,http://www.inchem.org/documents/icsc/icsc/eics0070.htm
- ^U.S. Department of Health and Human Services."How can phenol affect my health?"(PDF).Toxicological Profile for Phenol:24.
- ^Hanscha, Corwin; McKarns, Susan C; Smith, Carr J; Doolittle, David J (June 15, 2000). "Comparative QSAR evidence for a free-radical mechanism of phenol-induced toxicity".Chemico-Biological Interactions.127(1): 61–72.doi:10.1016/S0009-2797(00)00171-X.PMID10903419.
- ^"Medical Management Guidelines for Phenol (C6H6O)".Agency for Toxic Substances and Disease Registry.U.S. Department of Health and Human Services. October 21, 2014.Retrieved8 August2015.
- ^Brown, VKH; Box, VL; Simpson, BJ (1975). "Decontamination procedures for skin exposed to phenolic substances".Archives of Environmental Health.30(1): 1–6.doi:10.1080/00039896.1975.10666623.PMID1109265.
- ^Hunter, DM; Timerding, BL; Leonard, RB; McCalmont, TH; Schwartz, E (1992). "Effects of isopropyl alcohol, ethanol, and polyethylene glycol/industrial methylated spirits in the treatment of acute phenol burns".Annals of Emergency Medicine.21(11): 1303–7.doi:10.1016/S0196-0644(05)81891-8.PMID1416322.
- ^Pullin, TG; Pinkerton, MN; Johnston, RV; Kilian, DJ (1978). "Decontamination of the skin of swine following phenol exposure: a comparison of the relative efficacy of water versus polyethylene glycol/industrial methylated spirits".Toxicol Appl Pharmacol.43(1): 199–206.doi:10.1016/S0041-008X(78)80044-1.PMID625760.
External links
[edit]- International Chemical Safety Card 0070
- Phenol Material Safety Data Sheet
- National Pollutant Inventory: Phenol Fact Sheet
- NIOSH Pocket Guide to Chemical Hazards
- CDC - Phenol - NIOSH Workplace Safety and Health Topic
- IARC Monograph: "Phenol"
- Arcane Radio Trivia outlines competing uses for Phenol circa 1915






