Cephalosporin
| Cephalosporin | |
|---|---|
| Drug class | |
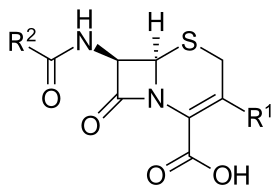 Core structure of the cephalosporins | |
| Class identifiers | |
| Use | Bacterial infection |
| ATC code | J01D |
| Biological target | Penicillin binding proteins |
| Clinical data | |
| Drugs.com | Drug Classes |
| External links | |
| MeSH | D002511 |
| Legal status | |
| In Wikidata | |

Thecephalosporins(sg./ˌsɛfələˈspɔːrɪn,ˌkɛ-,-loʊ-/[1][2]) are a class ofβ-lactam antibioticsoriginally derived from thefungusAcremonium,which was previously known asCephalosporium.[3]
Together withcephamycins,they constitute a subgroup of β-lactam antibiotics calledcephems.Cephalosporins were discovered in 1945, and first sold in 1964.[4]
Discovery[edit]
Theaerobicmoldwhichyieldedcephalosporin Cwas found in the sea near asewage outfallinSu Siccu,byCagliariharbourinSardinia,by theItalianpharmacologistGiuseppe Brotzuin July 1945.[5]
Structure[edit]
Cephalosporin contains a 6-membered dihydrothiazine ring. Substitutions at position 3 generally affect pharmacology; substitutions at position 7 affect antibacterial activity, but these cases are not always true.[6]
Medical uses[edit]
Cephalosporins can be indicated for theprophylaxisand treatment of infections caused bybacteriasusceptible to this particular form of antibiotic. First-generation cephalosporins are active predominantly againstGram-positivebacteria, such asStaphylococcusandStreptococcus.[7]They are therefore used mostly for skin and soft tissue infections and the prevention of hospital-acquired surgical infections.[8]Successive generations of cephalosporins have increased activity againstGram-negativebacteria, albeit often with reduced activity against Gram-positive organisms.[citation needed]
The antibiotic may be used for patients who are allergic to penicillin due to the differentβ-lactam antibioticstructure. The drug is able to be excreted in the urine.[7]
Side effects[edit]
Commonadverse drug reactions(ADRs) (≥ 1% of patients) associated with the cephalosporin therapy include: diarrhea, nausea, rash, electrolyte disturbances, and pain and inflammation at injection site. Infrequent ADRs (0.1–1% of patients) include vomiting, headache, dizziness, oral and vaginalcandidiasis,pseudomembranous colitis,superinfection,eosinophilia,nephrotoxicity,neutropenia,thrombocytopenia,andfever.[citation needed]
Allergic hypersensitivity[edit]
The commonly quoted figure of 10% of patients with allergic hypersensitivity topenicillinsand/orcarbapenemsalso having cross-reactivity with cephalosporins originated from a 1975 study looking at the original cephalosporins,[9]and subsequent "safety first" policy meant this was widely quoted and assumed to apply to all members of the group.[10]Hence, it was commonly stated that they are contraindicated in patients with a history of severe, immediate allergic reactions (urticaria,anaphylaxis,interstitial nephritis,etc.) to penicillins or carbapenems.[11]
The contraindication, however, should be viewed in the light of recent epidemiological work suggesting, for many second-generation (or later) cephalosporins, the cross-reactivity rate with penicillin is much lower, having no significantly increased risk of reactivity over the first generation based on the studies examined.[10][12]TheBritish National Formularypreviously issued blanket warnings of 10% cross-reactivity, but, since the September 2008 edition, suggests, in the absence of suitable alternatives, oralcefiximeor cefuroxime and injectable cefotaxime,ceftazidime,and ceftriaxone can be used with caution, but the use ofcefaclor,cefadroxil,cefalexin,andcefradineshould be avoided.[13]A 2012 literature review similarly finds that the risk is negligible with third- and fourth-generation cephalosporins. The risk with first-generation cephalosporins having similar R1 sidechains was also found to be overestimated, with the real value closer to 1%.[14]
MTT side chain[edit]
Several cephalosporins are associated withhypoprothrombinemiaand adisulfiram-like reaction with ethanol.[15][16]These includelatamoxef(moxalactam),cefmenoxime,cefoperazone,cefamandole,cefmetazole,andcefotetan.This is thought to be due to themethylthiotetrazoleside-chain of these cephalosporins, which blocks the enzymevitamin K epoxide reductase(likely causing hypothrombinemia) andaldehyde dehydrogenase(causing alcohol intolerance).[17]Thus, consumption of alcohol after taking these cephalosporin orally or intravenously is contraindicated, and in severe cases can lead to death.[18]The methylthiodioxotriazine sidechain found inceftriaxonehas a similar effect. Cephalosporins without these structural elements are believed to be safe with alcohol.[19]
Mechanism of action[edit]
Cephalosporins arebactericidaland, like other β-lactam antibiotics, disrupt the synthesis of thepeptidoglycanlayer forming the bacterialcell wall.The peptidoglycan layer is important for cell wall structural integrity. The final transpeptidation step in the synthesis of the peptidoglycan is facilitated bypenicillin-binding proteins(PBPs). PBPs bind to the D-Ala-D-Ala at the end of muropeptides (peptidoglycan precursors) to crosslink the peptidoglycan. Beta-lactam antibiotics mimic the D-Ala-D-Ala site, thereby irreversibly inhibiting PBP crosslinking of peptidoglycan.[20]
Resistance[edit]
Resistanceto cephalosporin antibiotics can involve either reduced affinity of existing PBP components or the acquisition of a supplementary β-lactam-insensitive PBP. Compared to other β-lactam antibiotics (such as penicillins), they are less susceptible toβ-lactamases.Currently, someCitrobacter freundii,Enterobacter cloacae,Neisseria gonorrhoeae,andEscherichia colistrains are resistant to cephalosporins. SomeMorganella morganii,Proteus vulgaris,Providencia rettgeri,Pseudomonas aeruginosa,Serratia marcescensandKlebsiella pneumoniaestrains have also developed resistance to cephalosporins to varying degrees.[21][22]
Classification[edit]
The cephalosporin nucleus can be modified to gain different properties. Cephalosporins are sometimes grouped into "generations" by theirantimicrobialproperties.[citation needed]
The first cephalosporins were designated first-generation cephalosporins, whereas, later, more extended-spectrumcephalosporins were classified as second-generation cephalosporins. Each newer generation has significantly greater Gram-negative antimicrobial properties than the preceding generation, in most cases with decreased activity against Gram-positive organisms. Fourth-generation cephalosporins, however, have true broad-spectrum activity.[23]
The classification of cephalosporins into "generations" is commonly practised, although the exact categorization is often imprecise. For example, the fourth generation of cephalosporins is not recognized as such in Japan.[citation needed]In Japan, cefaclor is classed as a first-generation cephalosporin, though in the United States it is a second-generation one; and cefbuperazone, cefminox, and cefotetan are classed as second-generation cephalosporins.
First generation[edit]
Cefalotin,cefazolin,cefalexin,cefapirin,cefradine,andcefadroxilare drugs belonging to this group.
Second generation[edit]
Cefoxitin,cefuroxime,cefaclor,cefprozil,andcefmetazoleare classed as second-generation cephems.
Third generation[edit]
Ceftazidime,ceftriaxone,andcefotaximeare classed as third-generation cephalosporins. Flomoxef and latamoxef are in a new, related class calledoxacephems.[24]
Fourth generation[edit]
Drugs included in this group arecefepimeandcefpirome.
Further generations[edit]
Some state that cephalosporins can be divided into five or even six generations, although the usefulness of this organization system is of limited clinical relevance.[25]
Naming[edit]
Most first-generation cephalosporins were originally spelled "ceph-" in English-speaking countries. This continues to be the preferred spelling in the United States, Australia, and New Zealand, while European countries (including the United Kingdom) have adopted theInternational Nonproprietary Names,which are always spelled "cef-". Newer first-generation cephalosporins and all cephalosporins of later generations are spelled "cef-", even in the United States.[citation needed]
Activity[edit]
There exist bacteria which cannot be treated with cephalosporins of generations first through fourth:[26]
- Listeria spp.
- Atypicals(includingMycoplasmaandChlamydia)
- MRSA
- Enterococci
Fifth-generation cephalosporins (e.g. ceftaroline) are effective against MRSA,Listeriaspp.,andEnterococcus faecalis.[27][26]
Overview table[edit]
Generation
|
Name | Approval status
|
Coverage
|
Description | ||
|---|---|---|---|---|---|---|
| Common | Alternate name or spelling | Brand | ||||
| (#) = noncephalosporins similar to generation # | H,human;V,veterinary;W,withdrawn;P,Pseudomonas;MR,methicillin-resistantStaphylococcus aureus;An,anaerobe | |||||
| 1 | Cefalexin | cephalexin | Keflex | HV | Gram-positive:Activity against penicillinase-producing, methicillin-susceptiblestaphylococciandstreptococci(though they are not the drugs of choice for such infections). No activity against methicillin-resistant staphylococci orenterococci.[citation needed]
Gram-negative:Activity againstProteus mirabilis,someEscherichia coli,andKlebsiella pneumoniae( "PEcK" ), but have no activity againstBacteroides fragilis,Pseudomonas,Acinetobacter,Enterobacter,indole-positiveProteus,orSerratia.[citation needed] | |
| Cefadroxil | cefadroxyl | Duricef | H | |||
| Cefazolin | cephazolin | Ancef, Kefzol | H | |||
| Cefapirin | cephapirin | Cefadryl | V | |||
| Cefacetrile | cephacetrile | |||||
| Cefaloglycin | cephaloglycin | |||||
| Cefalonium | cephalonium | |||||
| Cefaloridine | cephaloradine | |||||
| Cefalotin | cephalothin | Keflin | ||||
| Cefatrizine | ||||||
| Cefazaflur | ||||||
| Cefazedone | ||||||
| Cefradine | cephradine | Velosef | ||||
| Cefroxadine | ||||||
| Ceftezole | ||||||
| 2 | Cefuroxime | Altacef, Zefu, Zinnat, Zinacef, Ceftin, Biofuroksym,[28]Xorimax | H | Gram-positive:Less than first-generation.[citation needed]
Gram-negative:Greater than first-generation: HENHaemophilus influenzae,Enterobacter aerogenesand someNeisseria+ the PEcK described above.[citation needed] | ||
| Cefprozil | cefproxil | Cefzil | H | |||
| Cefaclor | Ceclor, Distaclor, Keflor, Raniclor | H | ||||
| Cefonicid | Monocid | |||||
| Cefuzonam | ||||||
| Cefamandole | W | |||||
| (2) | Cefoxitin | Mefoxin | H | An | Cephamycinssometimes grouped with second-generation cephalosporins | |
| Cefotetan | Cefotan | H | An | |||
| Cefmetazole | Zefazone | An | ||||
| Cefminox | ||||||
| Cefbuperazone | ||||||
| Cefotiam | Pansporin | |||||
| Loracarbef | Lorabid | Thecarbacephemanalog of cefaclor | ||||
| 3 | Cefdinir | Sefdin, Zinir, Omnicef, Kefnir | H | Gram-positive:Some members of this group (in particular, those available in an oral formulation, and those with antipseudomonal activity) have decreased activity against gram-positive organisms.
Activity against staphylococci and streptococci is less with the third-generation compounds than with the first- and second-generation compounds.[29] Gram-negative:Third-generation cephalosporins have a broad spectrum of activity and further increased activity against gram-negative organisms. They may be particularly useful in treatinghospital-acquired infections,although increasing levels of extended-spectrum beta-lactamases are reducing the clinical utility of this class of antibiotics. They are also able to penetrate thecentral nervous system,making them useful against meningitis caused by pneumococci, meningococci,H. influenzae,and susceptibleE. coli,Klebsiella,and penicillin-resistantN. gonorrhoeae.Since August 2012, the third-generation cephalosporin, ceftriaxone, is the only recommended treatment for gonorrhea in the United States (in addition to azithromycin or doxycycline for concurrentChlamydiatreatment). Cefixime is no longer recommended as a first-line treatment due to evidence of decreasing susceptibility.[30] | ||
| Ceftriaxone | Rocephin | H | ||||
| Ceftazidime | Meezat, Fortum, Fortaz | H | P | |||
| Cefixime | Fixx, Zifi, Suprax | H | ||||
| Cefpodoxime | Vantin, PECEF, Simplicef | HV | ||||
| Ceftiofur | Naxcel, Excenel | HV | ||||
| Cefotaxime | Claforan | H | ||||
| Ceftizoxime | Cefizox | H | ||||
| Cefditoren | Zostom-O | H | ||||
| Ceftibuten | Cedax | H | ||||
| Cefovecin | Convenia | V | ||||
| Cefdaloxime | ||||||
| Cefcapene | ||||||
| Cefetamet | ||||||
| Cefmenoxime | ||||||
| Cefodizime | ||||||
| Cefpimizole | ||||||
| Cefteram | ||||||
| Ceftiolene | ||||||
| Cefoperazone | Cefobid | W[31] | P | |||
| (3) | Latamoxef | moxalactam | W[31] | Anoxacephemsometimes grouped with third-generation cephalosporins | ||
| 4 | Cefepime | Maxipime | H | P | Gram-positive:They are extended-spectrum agents with similar activity against Gram-positive organisms as first-generation cephalosporins.[citation needed]
Gram-negative:Fourth-generation cephalosporins arezwitterionsthat can penetrate theouter membraneof Gram-negative bacteria.[32]They also have a greater resistance to β-lactamases than the third-generation cephalosporins. Many can cross theblood–brain barrierand are effective inmeningitis.They are also used againstPseudomonas aeruginosa.[citation needed] Cefiderocol has been called a fourth-generation cephalosporin by only one source as of November 2019.[33] | |
| Cefiderocol | Fetroja | H | ||||
| Cefquinome | V | |||||
| Cefclidine | ||||||
| Cefluprenam | ||||||
| Cefoselis | ||||||
| Cefozopran | ||||||
| Cefpirome | Cefrom | |||||
| (4) | Flomoxef | Anoxacephemsometimes grouped with fourth-generation cephalosporins | ||||
| 5 | Ceftaroline | H | MR | Ceftobiprolehas been described as "fifth-generation" cephalosporin,[34][35]though acceptance for this terminology is not universal. Ceftobiprole has anti-pseudomonalactivity andappearsto be less susceptible to development of resistance.Ceftarolinehas also been described as "fifth-generation" cephalosporin, but does not have the activity againstPseudomonas aeruginosaor vancomycin-resistant enterococci that ceftobiprole has.[36]Ceftolozaneis an option for the treatment of complicated intra-abdominal infections and complicated urinary tract infections. It is combined with theβ-lactamaseinhibitortazobactam,as multi-drug resistant bacterial infections will generally show resistance to allβ-lactam antibioticsunless this enzyme is inhibited.[37][38][39][40][41] | ||
| Ceftolozane | Zerbaxa | H | ||||
| Ceftobiprole | MR | |||||
| ? | Cefaloram | These cephems have progressed far enough to be named, but have not been assigned to a particular generation.Nitrocefinis a chromogenic cephalosporin substrate, and is used for detection of β-lactamases.[citation needed] | ||||
| Cefaparole | ||||||
| Cefcanel | ||||||
| Cefedrolor | ||||||
| Cefempidone | ||||||
| Cefetrizole | ||||||
| Cefivitril | ||||||
| Cefmatilen | ||||||
| Cefmepidium | ||||||
| Cefoxazole | ||||||
| Cefrotil | ||||||
| Cefsumide | ||||||
| Ceftioxide | ||||||
| Cefuracetime | ||||||
| Nitrocefin | ||||||
History[edit]
Cephalosporin compounds were first isolated from cultures ofAcremonium strictumfrom a sewer inSardiniain 1948 by Italian scientistGiuseppe Brotzu.[42]He noticed these cultures produced substances that were effective againstSalmonella typhi,the cause oftyphoid fever,which had β-lactamase.Guy NewtonandEdward Abrahamat theSir William Dunn School of Pathologyat theUniversity of Oxfordisolatedcephalosporin C.The cephalosporin nucleus,7-aminocephalosporanic acid(7-ACA), was derived from cephalosporin C and proved to be analogous to the penicillin nucleus6-aminopenicillanic acid(6-APA), but it was not sufficiently potent for clinical use. Modification of the 7-ACA side chains resulted in the development of useful antibiotic agents, and the first agent,cefalotin(cephalothin), was launched byEli Lilly and Companyin 1964.[citation needed]
References[edit]
- ^"cephalosporin".Merriam-Webster.com Dictionary.
- ^"cephalosporin – definition of cephalosporin in English from the Oxford dictionary".OxfordDictionaries.com.Archived fromthe originalon 7 July 2012.Retrieved20 January2016.
- ^"cephalosporin"atDorland's Medical Dictionary
- ^Oxford Handbook of Infectious Diseases and Microbiology.OUP Oxford. 2009. p. 56.ISBN9780191039621.
- ^Tilli Tansey;Lois Reynolds, eds. (2000).Post Penicillin Antibiotics: From acceptance to resistance?.Wellcome Witnesses to Contemporary Medicine.History of Modern Biomedicine Research Group.ISBN978-1-84129-012-6.OL12568269M.WikidataQ29581637.
- ^Prince, A."Cephalosporins and vancomycin"(PDF).Columbia University. Archived fromthe original(PDF)on 19 August 2019.Retrieved15 October2022.
- ^ab"Cephalosporins – Infectious Diseases".Merck Manuals Professional Edition.Retrieved15 May2019.
- ^Pandey, Neelanjana; Cascella, Marco (2020)."Beta Lactam Antibiotics".StatPearls.StatPearls.PMID31424895.
- ^Dash, C. H. (1 September 1975). "Penicillin allergy and the cephalosporins".Journal of Antimicrobial Chemotherapy.1(suppl 3): 107–118.doi:10.1093/jac/1.suppl_3.107.PMID1201975.
- ^abPegler, Scott; Healy, Brendan (10 November 2007)."In patients allergic to penicillin, consider second and third generation cephalosporins for life threatening infections".The BMJ.335(7627): 991.doi:10.1136/bmj.39372.829676.47.PMC2072043.PMID17991982.
- ^Rossi S, editor.Australian Medicines Handbook2006. Adelaide: Australian Medicines Handbook; 2006.[page needed]
- ^Pichichero, Michael E (2006)."Cephalosporins can be prescribed safely for penicillin-allergic patients".The Journal of Family Practice.55(2): 106–12.PMID16451776.
- ^"5.1.2 Cephalosporins and other beta-lactams".British National Formulary(56 ed.). London:BMJ Publishing Group LtdandRoyal Pharmaceutical Society Publishing.September 2008. pp.295.ISBN978-0-85369-778-7.
- ^Campagna, JD; Bond, MC; Schabelman, E; Hayes, BD (May 2012). "The use of cephalosporins in penicillin-allergic patients: a literature review".The Journal of Emergency Medicine.42(5): 612–20.doi:10.1016/j.jemermed.2011.05.035.PMID21742459.
- ^Kitson, Trevor M. (May 1987). "The effect of cephalosporin antibiotics on alcohol metabolism: A review".Alcohol.4(3): 143–148.doi:10.1016/0741-8329(87)90035-8.PMID3593530.
- ^Shearer, M. J.; Bechtold, H.; Andrassy, K.; Koderisch, J.; McCarthy, P. T.; Trenk, D.; Jähnchen, E.; Ritz, E. (January 1988). "Mechanism of Cephalosporin-induced Hypoprothrombinemia: Relation to Cephalosporin Side Chain, Vitamin K Metabolism, and Vitamin K Status".The Journal of Clinical Pharmacology.28(1): 88–95.doi:10.1002/j.1552-4604.1988.tb03106.x.PMID3350995.S2CID30591177.
- ^Stork CM (2006)."Antibiotics, antifungals, and antivirals".In Nelson LH, Flomenbaum N, Goldfrank LR, Hoffman RL, Howland MD, Lewin NA (eds.).Goldfrank's toxicologic emergencies.New York: McGraw-Hill. p. 847.ISBN978-0-07-143763-9.
- ^Ren, Shiyan; Cao, Yuxia; Zhang, Xiuwei; Jiao, Shichen; Qian, Songyi; Liu, Peng (2014)."Cephalosporin Induced Disulfiram-Like Reaction: A Retrospective Review of 78 Cases".International Surgery.99(2): 142–146.doi:10.9738/INTSURG-D-13-00086.1.ISSN0020-8868.PMC3968840.PMID24670024.
- ^Mergenhagen, Kari A.; Wattengel, Bethany A.; Skelly, Megan K.; Clark, Collin M.; Russo, Thomas A. (21 February 2020)."Fact versus Fiction: a Review of the Evidence behind Alcohol and Antibiotic Interactions".Antimicrobial Agents and Chemotherapy.64(3): e02167-19.doi:10.1128/aac.02167-19.PMC7038249.PMID31871085.
- ^Tipper, D J; Strominger, J L (October 1965)."Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine".Proceedings of the National Academy of Sciences of the United States of America.54(4): 1133–1141.Bibcode:1965PNAS...54.1133T.doi:10.1073/pnas.54.4.1133.ISSN0027-8424.PMC219812.PMID5219821.
- ^"Cephalosporin spectrum of resistance".Retrieved1 July2012.
- ^Sutaria, Dhruvitkumar S.; Moya, Bartolome; Green, Kari B.; Kim, Tae Hwan; Tao, Xun; Jiao, Yuanyuan; Louie, Arnold; Drusano, George L.; Bulitta, Jürgen B. (25 May 2018)."First Penicillin-Binding Protein Occupancy Patterns of β-Lactams and β-Lactamase Inhibitors in Klebsiella pneumoniae".Antimicrobial Agents and Chemotherapy.62(6): e00282-18.doi:10.1128/AAC.00282-18.PMC5971569.PMID29712652.
- ^"Cephalosporins – Infectious Diseases – Merck Manuals Professional Edition".Merck Manuals Professional Edition.Retrieved14 June2018.
- ^Narisada, Masayuki; Tsuji, Teruji (1990). "1-Oxacephem Antibiotics".Recent Progress in the Chemical Synthesis of Antibiotics.pp. 705–725.doi:10.1007/978-3-642-75617-7_19.ISBN978-3-642-75619-1.
- ^"Case Based Pediatrics Chapter".
- ^abBui, Toai; Preuss, Charles V. (2023),"Cephalosporins",StatPearls,Treasure Island (FL): StatPearls Publishing,PMID31855361,retrieved2 June2023
- ^Duplessis, C.; Crum-Cianflone, N. F. (2011)."Ceftaroline: A New Cephalosporin with Activity against Methicillin-Resistant Staphylococcus aureus (MRSA)".Clinical Medicine Reviews in Therapeutics.3:a2466.doi:10.4137/CMRT.S1637.PMC3140339.PMID21785568.
- ^Jędrzejczyk, Tadeusz."Internetowa Encyklopedia Leków".leki.med.pl. Archived fromthe originalon 7 October 2007.Retrieved3 March2007.
- ^Scholar, Eric M.; Scholar, Eric Michael; Pratt, William B. (2000).The Antimicrobial Drugs.Oxford University Press. p. 108.ISBN978-0-19-512528-3.
- ^Centers for Disease Control and Prevention (10 August 2012)."Update to CDC's Sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections".Morbidity and Mortality Weekly Report.61(31): 590–594.PMID22874837.
- ^abArumugham, VB; Gujarathi, R; Cascella, M (January 2021)."Third Generation Cephalosporins".PMID31751071.
- ^Richard L Sweet; Ronald S. Gibbs (1 March 2009).Infectious Diseases of the Female Genital Tract.Lippincott Williams & Wilkins. pp. 403–.ISBN978-0-7817-7815-2.Retrieved8 September2010.
- ^"CHEBI:140376 – cefiderocol".ebi.ac.uk.EMBL-EBI.Retrieved22 November2019.
- ^Widmer AF (March 2008)."Ceftobiprole: a new option for treatment of skin and soft-tissue infections due to methicillin-resistantStaphylococcus aureus"(PDF).Clin. Infect. Dis.46(5): 656–658.doi:10.1086/526528.PMID18225983.
- ^Kosinski, Mark A.; Joseph, Warren S. (July 2007). "Update on the Treatment of Diabetic Foot Infections".Clinics in Podiatric Medicine and Surgery.24(3): 383–396.doi:10.1016/j.cpm.2007.03.009.PMID17613382.
- ^Kollef MH (December 2009). "New antimicrobial agents for methicillin-resistantStaphylococcus aureus".Crit Care Resusc.11(4): 282–6.PMID20001879.
- ^Takeda, S; Nakai, T; Wakai, Y; Ikeda, F; Hatano, K (2007)."In vitro and in vivo activities of a new cephalosporin, FR264205, against Pseudomonas aeruginosa".Antimicrobial Agents and Chemotherapy.51(3): 826–30.doi:10.1128/AAC.00860-06.PMC1803152.PMID17145788.
- ^Toda, A; Ohki, H; Yamanaka, T; Murano, K; Okuda, S; Kawabata, K; Hatano, K; Matsuda, K; Misumi, K; Itoh, K; Satoh, K; Inoue, S (2008)."Synthesis and SAR of novel parenteral anti-pseudomonal cephalosporins: Discovery of FR264205".Bioorganic & Medicinal Chemistry Letters.18(17): 4849–52.doi:10.1016/j.bmcl.2008.07.085.PMID18701284.
- ^Sader, H. S.; Rhomberg, P. R.; Farrell, D. J.; Jones, R. N. (2011)."Antimicrobial activity of CXA-101, a novel cephalosporin tested in combination with tazobactam against Enterobacteriaceae, Pseudomonas aeruginosa, and Bacteroides fragilis strains having various resistance phenotypes".Antimicrobial Agents and Chemotherapy.55(5): 2390–4.doi:10.1128/AAC.01737-10.PMC3088243.PMID21321149.
- ^Craig, W. A.; Andes, D. R. (2013)."In vivo activities of ceftolozane, a new cephalosporin, with and without tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae, including strains with extended-spectrum β-lactamases, in the thighs of neutropenic mice".Antimicrobial Agents and Chemotherapy.57(4): 1577–82.doi:10.1128/AAC.01590-12.PMC3623364.PMID23274659.
- ^Zhanel, G. G.; Chung, P; Adam, H; Zelenitsky, S; Denisuik, A; Schweizer, F; Lagacé-Wiens, P. R.; Rubinstein, E; Gin, A. S.; Walkty, A; Hoban, D. J.; Lynch Jp, 3rd; Karlowsky, J. A. (2014)."Ceftolozane/tazobactam: A novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli".Drugs.74(1): 31–51.doi:10.1007/s40265-013-0168-2.PMID24352909.S2CID44694926.
{{cite journal}}:CS1 maint: numeric names: authors list (link) - ^Podolsky, Daniel K. (1998).Cures out of Chaos.CRC Press.ISBN978-1-4822-2973-8.[page needed]


