Cholestasis
This articleneeds morereliable medical referencesforverificationor relies too heavily onprimary sources.(March 2019) |  |
| Cholestasis | |
|---|---|
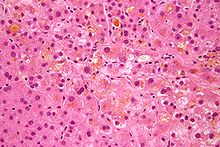 | |
| Micrographshowingbile(yellow) stasis in liver tissue, i.e. cholestasis.H&E stain. | |
| Specialty | Gastroenterology |
Cholestasisis a condition where the flow ofbilefrom theliverto theduodenumis impaired.[1]The two basic distinctions are:[1]
- obstructive type of cholestasis, where there is a mechanical blockage in the duct system that can occur from agallstoneormalignancy,and
- metabolic type of cholestasis, in which there are disturbances in bile formation that can occur because ofgenetic defectsor acquired as a side effect of many medications.
Classification is further divided into acute or chronic and extrahepatic or intrahepatic.
Signs and symptoms[edit]
The signs and symptoms of cholestasis vary according to the cause. In case of sudden onset, the disease is likely to be acute, while the gradual appearance of symptoms suggests chronic pathology.[2]In many cases, patients may experience pain in the abdominal area. Localization of pain to theupper right quadrantcan be indicative ofcholecystitisorcholedocholithiasis,which can progress to cholestasis.[3][4]
Pruritusor itching is often present in many patients with cholestasis.[5]Patients may present with visible scratch marks as a result of scratching.[2]Pruritus is often misdiagnosed as a dermatological condition, especially in patients that do not havejaundiceas an accompanying symptom.[2]In a typical day, pruritus worsens as the day progresses, particularly during the evening time.[6]Overnight, pruritus dramatically improves. This cycle can be attributed to an increase in the concentration of biliary elements during the day due to food consumption, and a decline at night.[2]Pruritus is mostly localized to the limbs, but can also be more generalized.[6]The efficacy of naltrexone for cholestatic pruritus suggests involvement of the endogenous opioid system.
Many patients may experiencejaundiceas a result of cholestasis.[7]This is usually evident after physical examination as yellow pigment deposits on theskin,in theoral mucosa,orconjunctiva.[2][8]Jaundice is an uncommon occurrence in intrahepatic (metabolic) cholestasis, but is common in obstructive cholestasis. The majority of patients with chronic cholestasis also experiencefatigue.[9]This is likely a result of defects in thecorticotrophinhormone axis or other abnormalities withneurotransmission.[2]Some patients may also havexanthomas,which are fat deposits that accumulate below the skin.[10]These usually appear waxy and yellow, predominantly around the eyes and joints.[11]This condition results from an accumulation of lipids within the blood.[12]If gallstones prevent bile flowing from the pancreas to the small intestine, it can lead to gallstonepancreatitis.Physical symptoms include nausea, vomiting, and abdominal pain.
Bileis required for the absorption offat-soluble vitamins.[13]As such, patients with cholestasis may present with a deficiency in vitamins A, D, E, or K due to a decline in bile flow.[14]Patients with cholestasis may also experience pale stool and dark urine.[15]
Causes[edit]
Possible causes:
- pregnancy
- androgens
- birth control pills
- antibiotics (such as TMP/SMX)
- abdominal mass(e.g.cancer)
- pediatric liver diseases[15]
- biliarytrauma
- congenital anomalies of the biliary tract
- gallstones
- biliary dyskinesia
- acute hepatitis[17]
- cystic fibrosis
- primary biliary cholangitis,[15]an autoimmune disorder
- primary sclerosing cholangitis,[15]associated withinflammatory bowel disease
- some drugs (e.g.flucloxacillinanderythromycin)[18]: 208
- secondary syphilis,albeit rarely[19][20]
Drugs such asgold salts,nitrofurantoin,anabolic steroids,sulindac,chlorpromazine,erythromycin,prochlorperazine,cimetidine,estrogen,and statins can cause cholestasis and may result in damage to theliver.[18]: 208 [21][22]
Drug-induced cholestasis[edit]
Acute and chronic cholestasis can be caused by certain drugs or their metabolites. Drug-induced cholestasis (DIC) falls under drug-induced liver injury (DILI), specifically the cholestatic or mixed type.[23][24]While some drugs (e.g.,acetaminophen) are known to cause DILI in a predictable dose-dependent manner (intrinsic DILI), most cases of DILI areidiosyncratic,i.e., affecting only a minority of individuals taking the medication.[25][26]Seventy-three percent of DIC cases can be attributed to a single prescription medication, commonlyantibiotics&antifungals,anti-diabetics,anti-inflammatory,&cardiovascular drugs,psychotropicdrugs.[24][27]The exact pathomechanism may vary for different drugs and requires further elucidation.[27]
Typical symptoms of DIC includepruritusandjaundice,nausea,fatigue, and dark urine, which usually resolve after discontinuation of the offending medication.[24][28]
Clinically, DIC can manifest as acute bland (pure) cholestasis, acute cholestatichepatitis,secondary sclerosing cholangitis(involving bile duct injury), orvanishing bile duct syndrome(loss of intrahepatic bile ducts).[29][23][18]: 17 [30]Bland cholestasis occurs when there is obstruction to bile flow in the absence of inflammation or biliary and hepatic injury, whereas these features are present in cholestatic hepatitis.[29][18]: 17 Bland cholestasis is almost always caused byanabolic steroidsorestrogencontraceptive use,[31]while many drugs may cause cholestatic hepatitis, includingpenicillins,sulfonamides,rifampin,cephalosporins,fluoroquinolones,tetracyclines,andmethimazole,among others.[28][29]
Antibioticsandantifungalsthat commonly cause DIC arepenicillins,macrolides,trimethoprim/sulfamethoxazole,and tetracyclines.[29]Due to its clavulanic acid component, penicillinamoxicillin-clavulanateis the most common culprit of cholestatic liver injury.[29]Flucloxacillin,which is commonly prescribed in the UK, Sweden, and Australia, is another penicillin frequently implicated in DIC. Cholestasis induced by penicillins usually resolves after withdrawal.[29]Macrolideswith cholestatic potential include erythromycin, clarithromycin, and azithromycin, and prognosis is likewise favorable with these drugs.[29]Trimethoprim/sulfamethoxazole(via its sulfonamide component) is the fourth most common antibiotic responsible for DILI in North America. However, DIC is comparatively less common with low-dose tetracyclines like doxycycline.[29]Other cholestatic antimicrobials include the antifungalterbinafine,notable for its potential to cause life-threatening cholestatic injury, andquinolones(ciprofloxacin, levofloxacin), which have been linked to cholestatic hepatitis and vanishing bile duct syndrome.[29]
Among psychotropic drugs,chlorpromazineis known to cause cholestatic hepatitis.Tricyclic antidepressants(imipramine, amitriptyline) andSSRIs(duloxetine) causing cholestasis have also been reported.[29]Anti-inflammatory drugs with cholestatic potential include the immunosuppressantazathioprine,which has been reported to cause fatal cholestatic hepatitis, and the NSAIDdiclofenac.[29]
Rare causes of cholestasis[edit]
The causes of cholestasis are diverse, and some feature more prominently than others. Some rare causes includeprimary sclerosing cholangitis,primary biliary cholangitis,familial intrahepatic cholestasis,Alagille syndrome,sepsis,total parenteral nutrition-based cholestasis, benign recurrent intrahepatic cholestasis,biliary atresia,andintrahepatic cholestasis of pregnancy.
Primary biliary cholangitis[edit]
Chronic cholestasis occurs inprimary biliary cholangitis(PBC). PBC is a progressive autoimmune liver disease in which small intrahepatic bile ducts are selectively destroyed, leading to cholestasis, biliary fibrosis, cirrhosis, and eventually liver failure that requires transplantation.[32][33][34]Prevalence of PBC ranges from 19 to 402 cases/million depending on geographic location,[32]with a 9:1 female preponderance[35]and median ages of diagnosis of 68.5 years for females and 54.5 years for males.[36]
At diagnosis, 50% of PBC patients are asymptomatic, indicative of an early stage of disease, while another 50% report fatigue and daytime sleepiness. Other symptoms include pruritus and skin lesions, and in prolonged cholestasis, malabsorption and steatorrhea leading to fat-soluble vitamin deficiency. Disease progression is accompanied by intensifying portal hypertension and hepatosplenomegaly. Clinically, diagnosis generally requires a 1:40 or greater titer ofanti-mitochondrial antibody(AMA) againstPDC-E2and elevatedalkaline phosphatasepersisting for 6+ months.[32]
Ursodeoxycholic acid(UDCA) is an FDA-approved first-line treatment for PBC. At moderate doses, UDCA has been demonstrated to slow disease progression and improve transplant-free survival. A complete response is achieved in 25-30% patients, and similar survival as the general population is expected in 2/3 of patients on UDCA. For the 1/3 non-responders,obeticholic acid(OCA) is approved by the FDA as a second-line treatment.[32]
The precise etiology of PBC remains poorly understood, though a clearer picture is starting to emerge. A loss of immune tolerance is indicated by the presence of AMAs and autoreactive CD4+ and CD8+T cellstargetingcholangiocytesthat line the bile ducts.[33]Cholangiocytes are normally responsible for 40% of bile flow, mostly through secretion of bicarbonate into bile viaanion exchanger 2(AE2) on their apical membrane.[34]The resulting bicarbonate "umbrella" that forms over cholangiocytes provides protection from toxicbile salts.[34]However, in PBC there is repression of AE2 activity due to upregulation ofmiR-506.This results in decreased biliary bicarbonate secretion and consequently, cholestasis and injury to cholangiocytes by bile salts.[34][37]Injury may induce cholangiocytes to undergo apoptosis, and during this process, the unique way in which cholangiocytes handle the degradation ofPDC-E2(the E2 subunit of mitochondrial pyruvate dehydrogenase complex) may be a trigger for PSC. Specifically, PDC-E2 in apoptotic cholangiocytes undergo a covalent modification that may render them recognizable to antibodies and thereby trigger a break in self-tolerance.[34]The problem is compounded by cholangiocytes' peculiarly abundant expression ofHLA-II and HLA-I, as well as adhesion and chemoattractant molecules, which recruit aid in recruitment of mononuclear immune cells.[38][34]
Both genetic and environmental factors probably contribute to PBC pathogenesis.[33]Genetic predisposition is suggested by highconcordancebetween identical twins, higher incidence among relatives, and a strong association of disease with certain HLA variants.[33]Disease is likely triggered in the genetically predisposed by some environmental factor, such as pollutants, xenobiotics (e.g., chemicals in makeup), diet, drugs, stress, and infectious agents.Urinary tract infectionwithE. coliis a particularly strong risk factor for PBC. A possible explanation is that E. coli possess a similar PDC-E2 as humans which could trigger autoimmunity viamolecular mimicry.[34][38][33]
Primary sclerosing cholangitis[edit]
Chronic cholestasis is a feature in primary sclerosing cholangitis (PSC). PSC is a rare and progressive cholestatic liver disease characterized by narrowing, fibrosis, and inflammation of intrahepatic or extrahepatic bile ducts, leading to reduced bile flow or formation (i.e., cholestasis).[39][40]The pathogenesis of PSC remains unclear but probably involves a combination of environmental factors and genetic predisposition.[40]Notably, 70-80% of patients with PSC are comorbid withinflammatory bowel disease(e.g.,ulcerative colitisorCrohn's colitis), suggesting there exists a link between the two.[39][41]
PSC predominantly affects males (60-70%) of 30–40 years of age.[40]The disease has an incidence is 0.4-2.0 cases/100,000 and a prevalence of 16.2 cases/100,000, making it a rare disease.[42][40]Nonetheless, PSC accounts for 6% of liver transplants in the US due to its eventual progression to end-stage liver disease, with a mean transplant-free survival of 21.3 year.[40]
Though 40-50% of patients are asymptomatic, commonly reported symptoms include abdominal pain in theright upper quadrant,pruritus,jaundice,fatigue,andfever.[39][40]The most common signs arehepatomegalyandsplenomegaly.[39]Prolonged cholestasis in PSC may cause fat-soluble vitamin deficiency leading to osteoporosis[39]
Diagnosis requires elevated serumalkaline phosphatasepersisting for at least 6 months and the presence of bile duct strictures oncholangiogram.[39][40]Unlikeprimary biliary cholangitis,PSC lacks a diagnostic autoantibody or reliable biomarker of disease progression.[40][39]Although a liver biopsy is not required for diagnosis, the characteristic histological finding is concentric periductal fibrosis resembling onion skin.[40]
PSC is associated with increased risk of several cancers, most notably, a 400 times greater risk forcholangiocarcinomacompared to the general population.[39]Patients with PSC also face elevated risk ofpancreaticandcolorectalcancer.[40]Therefore, regular screening is recommended.[39]
No drugs are currently approved for treating PSC specifically.[32]Although commonly given,ursodeoxycholic acidat moderate doses failed to improve transplant-free survival in randomized controlled trials.[40][39]Due to disease progression, 40% of patients eventually require liver transplantation, which has survival rates (91% at 1 year, 82% at 5 years, and 74% at 10 years).[40]However, the disease recurs in at least 25% of transplant recipients, particularly in those with IBD and an intact colon.[39]Clinical trials are underway for several novel therapies, includingobeticholic acid(a bile acid analogue),simtuzumab(a monoclonal antibody), and 24-norursodeoxycholic acid (a synthetic bile acid).[39]
Although the pathogenesis of PSC is poorly understood, three dominant theories have been proposed: 1) aberrant immune response, 2) increased intestinal permeability, and 3) dysbiosis of gut microbiota.[43]The first theory involves immune-mediated damage to bile ducts by T cells. In PSC,cholangiocytesandhepatocytesdisplay aberrant expression of adhesion molecules, which facilitate homing of intestinal T cells to the liver.[43][39]Additionally, intestinal microbiota may produce pathogen-associated molecular patterns that stimulatecholangiocytesandhepatic macrophagesto produceproinflammatory cytokines,which promote recruitment of immune cells to the bile ducts, fibrosis, cholangiocyte apoptosis and senescence, and ultimately destruction of the bile ducts.[39][34]In support of T cell involvement, certainhuman leukocyte antigen(HLA) variants are strongly associated with PSC risk.[39]Further evidence for genetic predisposition include the identification of 23 non-HLA susceptibility loci and a higher disease risk among siblings, though environmental factors appear to play a much greater role in pathogenesis.[41]
Another theory postulates that increased intestinal permeability contributes to PSC.Tight junctions,which normally maintain the integrity of the intestinal epithelium, may become disrupted in inflammation.[43]Leaky tight junctions could allow commensal bacteria and toxins to enter portal circulation and reach the liver, where they can trigger inflammation and fibrosis.[43]
The intestinal dysbiosis theory hypothesizes that yet unidentified environmental triggers (e.g., diet, medication, inflammation) reduce microbiota diversity and/or alter the population of specific species.[43]The resulting imbalance between primary and secondary bile acids may lead to PSC via the gut-liver axis.[43]The primary bile acidscholic acid(CA) andchenodeoxycholic acid(CDCA) are synthesized in the liver and undergo conjugation before being released into the small intestine to aid digestion.[41]In the distal ileum, 95% of these conjugated BAs are actively reabsorbed viaASBTbut 5% enter the colon and are converted by gut microbes into deconjugated secondary bile acids, predominantlydeoxycholic acid(DCA) andlithocholic acid(LCA).[44][41]DCA and LCA are then reabsorbed into portal circulation and reach the liver, where they serve as signaling molecules that maintain bile acid homeostasis.[41]Specifically, DCA and LCA and potent agonists offarnesoid X receptor(FXR) andTakeda G protein-coupled receptor 5(TGR5),[41]both of which mediate anti-inflammatory and cholangioprotective effects upon activation.[43]On cholangiocytes, TGR5 activation inducesCFTRto secrete chloride into bile ducts, which then drivesanion exchanger 2to secrete bicarbonate into bile canaliculi.[41]Bicarbonateserves to protect the apical surface of cholangiocytes from damage by bile acids.[41]On macrophages, activation of FXR and TGR5 inhibitsNF-κB,thereby reducing production of proinflammatory cytokines.[41]Therefore, it is hypothesized that a reduction in secondary bile acid production, as a result ofdysbiosis,could lead to bile duct damage via decreased activation of FXR and TGR5. Indeed, lower levels of secondary bile acids were found in PSC patients, but a causal relationship is yet to be confirmed.[45][46]
Familial intrahepatic cholestasis[edit]
Familial intrahepatic cholestasis (FIH) is a group of disorders that lead to intrahepatic cholestasis in children.[47]Most often, FIH occurs during the first year of life, with an incidence rate of 1/50,000 to 1/100,000.[48]There are three different versions of FIH, with each causing a different severity ofjaundice.Typically, children exhibit recurrent jaundice episodes, which eventually become permanent.[47]Diagnosis usually occurs by analyzing laboratory features, liver biopsy results, DNA/RNA sequences, and biliary lipid analysis.[47]The definitive treatment for FIH isliver transplantwhich usually results in a high recovery rate.[47]Each type of FIH is a result of a different mutation. The three genes thought to be involved includeAPT8B1,which encodes for the FIC1 protein.[49]TheABCB11gene encodes for the bile salt export pump (BSEP) protein, and theABCB4gene encodes for the multidrug resistance 3 (MDR3) protein.[49][50]BSEP and MDR3 are respectively responsible for transporting bile salt and phospholipid, two major constituents of bile, across the apical membrane of hepatocytes.[51]
Alagille syndrome[edit]
Alagille syndromeis an autosomal dominant disorder that impacts five systems, including the liver, heart, skeleton, face, and eyes.[47]In the early part of life (within the first three months), patients with Alagille syndrome exhibit conjugatedhyperbilirubinemia,severepruritus,and jaundice.[47]Bile duct obliteration usually worsens over time, causingcirrhosisof the liver and eventual failure.[47]Diagnosis usually occurs using the classic criteria by looking at changes associated with the five systems discussed earlier.[52]Like FIH, the definitive treatment is a liver transplant.[53]Almost all patients with Alagille syndrome have mutations of the genes involved in theNotch signaling pathway.Most have a mutation of theJAG1gene, while a small minority have a mutation of theNOTCH2gene.[54][55]
Sepsis[edit]
A variety of factors associated withsepsismay cause cholestasis. Typically, patients have conjugated hyperbilirubinemia andalkaline phosphatase(ALP) elevation but not to extreme levels.[56]Sepsis-induced cholestasis may occur due to increased serumlipopolysaccharidelevels.Lipopolysaccharidescan inhibit and down-regulate bile salt transporters in hepatocytes, thereby leading to cholestasis.[47]As such, in the case of sepsis, cholestasis occurs not as a result of impaired obstruction but rather the disruption of bile flow.Ischemicliver injury resulting from sepsis can also cause cholestasis. Importantly,jaundiceis not indicative of cholestasis in all cases. Widespreadhemolysisresulting from sepsis may releasebilirubin,thereby overwhelming bilirubin reabsorption and excretion mechanism.[47]
TPN-based cholestasis[edit]
Total parenteral nutrition(TPN) is given to patients with intestinal failure or a variety of other gastrointestinal problems.[47]Under normal settings, TPN causes a slight elevation of ALP levels. However, this does not indicate cholestasis alone.[47]In the case of TPN-induced cholestasis, there is an excessive elevation of ALP,gamma-glutamyltransferase(GGT), and conjugated bilirubin.[57]Without appropriate intervention, symptoms can quickly exacerbate, leading to livercirrhosisand failure.[47]Cholestasis arising from TPN has a diverse range of causes, including toxicity to TPN components, underlying disorders, or a lack of enteral nutrition.[47]Without enteral food consumption,gallbladderfunction is greatly inhibited, leading togallstoneformation, subsequent blockage, and eventually cholestasis.[47]Cholestasis resulting from TPN may also be a result of reduced bile flow from portal endotoxins.[47]With TPN, there is a reduction in gastrointestinal motility, immunity, with an increase in permeability.[47]These changes facilitate bacteria growth and increase the amount of circulating endotoxin. Moreover, given that patients using TPN often have underlying health problems, drugs being used with known liver toxicity may also cause cholestasis.Lipidsin TPN may cause cholestasis and liver damage by overwhelming clearage mechanisms.[58]Intravenous glucose can also cause cholestasis as a result of increasedfatty acid synthesisand decreased breakdown, which facilitates the accumulation of fats.[59]
Intrahepatic cholestasis of pregnancy (obstetric cholestasis)[edit]
Intrahepatic cholestasis of pregnancy(ICP) is an acute cause of cholestasis that manifests most commonly in the thirdtrimesterof pregnancy.[15]It affects 0.5–1.5% of pregnancies in Europe and the US and up to 28% in women ofMapucheethnicity inChile.[60]ICP is characterized by severepruritusand elevated serum levels ofbile acidsas well astransaminasesandalkaline phosphatase.[61]These signs and symptoms resolve on their own shortly after delivery, though they may reappear in subsequent pregnancies for 45–70% of women.[62]In the treatment of ICP, current evidence suggestsursodeoxycholic acid(UDCA), a minor secondary bile acid in humans, is the most effective drug for reducing pruritus and improving liver function.[61]
The etiology of ICP is multifactorial and likely involves hormonal, genetic, and environmental factors. Several observations suggestestrogenplays a major role: ICP begins in the third trimester, when estrogen levels are highest, resolves after estrogen levels return to normal post-delivery, and occurs with higher incidence inmultiple pregnancies,where estrogen levels are more elevated than usual.[62][63]Although estrogen's exact pathomechanism in ICP remains unclear, several explanations have been offered. Estrogen may induce a decrease in the fluidity of the hepaticsinusoidalmembrane, leading to a decrease in the activity of basolateralNa+/K+-ATPase.[62][64][65]A weaker Na+ gradient results in diminished sodium-dependent uptake of bile acids from venous blood into hepatocytes by thesodium/bile acid cotransporter.[62][66]More recent evidence suggests that estrogen promotes cholestasis via its metaboliteestradiol-17-β-D-glucuronide(E2).[60][62]E2 secreted into the canaliculi byMRP2was found to repress the transcription of bile salt export pump (BSEP),[67][60][68]the apicalABC transporterresponsible for exporting monoanionic conjugated bile acids from hepatocytes intobile canaliculi.[67]E2 was also found to upregulate miR-148a, which represses expression of thepregnane X receptor(PXR).[67]PXR is a nuclear receptor in hepatocytes that senses intracellular bile acid concentrations and regulates gene expression accordingly to increase bile efflux.[69]
Genetic predisposition for ICP is suggested by familial and regional clustering of cases.[62][60]Several studies have implicated heterozygous mutations of the genesABCB11andABCB4in ICP, which respectively encode the canalicular transport proteins BSEP andmultidrug resistance protein 3(MDR3).[70][60]MDR3 is responsible for exportingphosphatidylcholine,the majorlipidcomponent of bile, into bile canaliculi where it formsmicelleswith bile salts to prevent the latter from damaging luminal epithelium. Bile flow requires canalicular secretion of both bile salts and phosphatidylcholine.[70]MDR3 mutations are an established predisposing factor, found in 16% of ICP cases.[60][71]More recently, studies have demonstrated involvement of BSEP mutations in at least 5% of cases.[70]The V444A polymorphism ofABCB11in particular may lead to ICP by causing a reduction in hepatic BSEP expression and consequently decreased bile salt export.[60]Other notable mutations identified in ICP patients include ones in thefarnesoid X receptor(FXR), a nuclear receptor in hepatocytes which activates transcription of MDR3 and BSEP upon binding intracellular bile acids, thereby increasing canalicular bile efflux.[60][72][68]
Mechanism[edit]
Bileis secreted by the liver to aid in thedigestionoffats.Bile formation begins inbile canaliculithat form between two adjacent surfaces of liver cells (hepatocytes) similar to the terminal branches of a tree. The canaliculi join each other to form larger and larger structures, sometimes referred to as thecanals of Hering,which themselves join to form small bile ductules that have an epithelial surface. The ductules join to formbile ductsthat eventually form either the right main hepatic duct that drains the right lobe of the liver, or the left main hepatic duct draining the left lobe of the liver. The two ducts join to form thecommon hepatic duct,which in turn joins thecystic ductfrom thegall bladder,to give thecommon bile duct.This duct then enters the duodenum at theampulla of Vater.In cholestasis, bile accumulates in the hepaticparenchyma.[73]
One of the most common causes of extrahepatic, or obstructive cholestasis, is biliary obstruction. This is better known ascholedocholithiasiswhere gallstones become stuck in the common bile duct.

Mechanisms of drug-induced cholestasis[edit]
Drugs may induce cholestasis by interfering with 1) hepatic transporters, 2)bile canaliculidynamics, and/or 3) cell structure and protein localization.[24][74]Hepatictransportersare essential for maintaining enterohepatic bile flow and bile acid homeostasis.[75]Therefore, their direct inhibition by certain drugs may lead to cholestasis. Relevant transporters implicated includeBSEP,MDR3,MRP2-4,andNTCP.[24][65]
Cholestasis can result fromcompetitive inhibitionof BSEP by several drugs, includingcyclosporine A,rifampicin,nefazodone,glibenclamide,troglitazone,andbosentan.[27][74]BSEP is the main transporter in hepatocytes responsible for exportingbile saltsacross the apical membrane intobile canaliculi.Therefore, inhibiting BSEP should cause cytotoxic bile salts to accumulate in hepatocytes, leading to liver injury and impaired bile flow.[27]Indeed, there is a strong association between BSEP inhibition and cholestasis in humans, and BSEP inhibitors are shown to induce cholestasisin vitro.[74]However, hepatocytes have safety mechanisms that can compensate for impaired canalicular bile efflux.[74]In response to cholestasis, MRP3 and MRP4 on thebasolateral membraneare upregulated to allow efflux of accumulated bile salts into portal blood. Similarly, MRP2 can accommodate additional bile flow across the apical membrane in cholestatic conditions.[65][74]These compensatory mechanisms explain why some BSEP inhibitors do not cause cholestasis.[27][74]On the contrary, contrast, drugs that inhibit both MRP3/4 and BSEP (e.g., rifampicin, troglitazone, bosentan) pose greater risk for cholestasis[65]
MDR3 is another key canalicular efflux transporter that is the target of inhibition by certain drugs. MDR3 secretesphosphatidylcholineinto bile canaliculi, where it formmicelleswith bile salts to dissolvecholesterolas well as protect hepatocyte and cholangiocytes from damage by bile salts.[24]MDR3 inhibition leads to lowphospholipidconcentrations in bile that damages cholangiocytes and leads to cholestasis.[76]Antifungal azoles suchitraconazolehave been shown to inhibit both MDR3 and BSEP, thus giving them higher cholestatic potential.[76]Other MDR3-inhibiting drugs arechlorpromazine,imipramine,haloperidol,ketoconazole,saquinavir,clotrimazole,ritonavir,andtroglitazone.[27]
Another target for inhibition, MRP2 is an apical efflux transporter that mainly exportsbilirubin glucuronideandglutathioneinto bile. However, MRP2 is also the preferential route of export for certain sulfated conjugated BAs (taurolithocholic acidand glycolithocholic acid), so its inhibition could contribute to cholestasis.[65]
On the hepatocyte basolateral membrane, Na+-taurocholate cotransporting peptide (NTCP) is the major transporter of conjugated bile acids.[74]Enterohepatic bile flow requires the concerted activity of both NTCP and BSEP, which form the major route by which BAs enter and exit hepatocytes respectively.[65]Therefore, NTCP inhibitors, such as cyclosporine A,ketoconazole,propranolol,furosemide,rifamycin,saquinavir,andritonavir,should theoretically cause cholestasis by decreasing hepatocyte BA uptake.[74]However, no relationship was found between NTCP inhibition and DIC risk,[74]possibly because basolateral sodium-independentOATPscan partially compensate for bile salt uptake.[74]Therefore, NTCP inhibition alone seems to be insufficient for causeing cholestasis.[74]Indeed, the cholestatic effect ofcyclosporine Arelies on its inhibition of both NTCP and the compensatoryOATP1B1.[74][76]
In addition to direct inhibition, drugs can also induce cholestasis by promoting downregulation and internalization of transporters. For example, cyclosporine A in rats was shown to induce BSEP internalization in addition to inhibition. Furthermore, human hepatocytes showed decreased expression of BSEPmRNAand protein following long-term exposure to metformin andtamoxifen,neither of which are direct BSEP inhibitors.[74]
Bile canaliculi dynamics refers to the contractile motion of bile canaliculi (ducts) required for bile flow. Cholestasis can result when drugs constrict or dilate bile canaliculi. Constrictors include chlorpromazine,nefazodone,troglitazone,perhexiline,metformin,cyclosporin A. These drugs activate the RhoA/Rho-kinase pathway, which inhibitsmyosin light chain phosphatase(MLCP), and in turn, increasesmyosin light chainphosphorylation byMLC kinaseleading to constriction of bile canaliculi. Drugs that dilate canaliculi work by inhibiting MLCK or RhoA/Rho-kinase and includediclofenac,bosentan,entacapone,tacrolimus,cimetidine,andflucloxacillin.[65][24]Constriction is more serious than dilation, as the former causes irreversible cell damage and death.[24]
Minor mechanisms that may contribute to DIC include aberrantparacellularpermeability, membrane fluidity, and transporter localization.[24]Tight junctionsnormally seal the gap between hepatocytes to prevent bile from diffusing out of the canaliculi. If a drug causes internalization of hepatocyte tight junctions, like rifampicin does in mice, bile flow may become impaired due to paracellular leakage.[65]Membrane fluidity can affect bile flow by regulating the activity of hepatocyteNa+/K+-ATPase,which maintains the inwardly-directed Na+ gradient that drives BA uptake by apicalNTCP.[65][74]In rats,cyclosporine Awas found to increase canalicular membrane fluidity and consequently reduce bile secretion. Bile flow was similarly reduced in rats as a result of alterations to basolateral membrane fluidity byethinylestradiolandchlorpromazine.[65]Lastly, some agents (rimpaficin and17β-estradiol) were shown to hinder proper localization of hepatocyte transporters by interfering with the microtubules required for their insertion into plasma membranes.[24]
Diagnosis[edit]
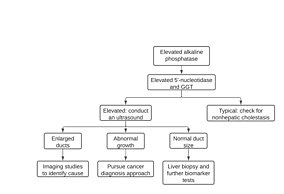
Cholestasis can be suspected when there is an elevation of both5'-nucleotidaseandALPenzymes.[77]With a few exceptions, the optimal test for cholestasis would be elevations of serumbile acidlevels.[78]However, this is not normally available in most clinical settings necessitating the use of other biomarkers. If 5' nucleosidase and ALP enzymes are elevated,imaging studiessuch ascomputed tomography(CT) scan,ultrasound,andmagnetic resonance imaging(MRI) are used to differentiate intrahepatic cholestasis from extrahepatic cholestasis.[77]Additional imaging, laboratory testing, and biopsies might be conducted to identify the cause and extent of cholestasis.[77]
Biomarkers[edit]
ALP enzymes are found abundantly within thebile canaliculiandbile.If a duct is obstructed, tight junctions permit migration of the ALP enzymes until the polarity is reversed and the enzymes are found on the whole of the cell membrane.[77]Serum ALP levels exceeding 2-3 times the upper baseline value may be due to a variety of liver diseases.[79]However, an elevation that exceeds 10 times the upper baseline limit is strongly indicative of either intrahepatic or extrahepatic cholestasis and requires further investigation.[79]Cholestasis can be differentiated from other liver disorders by measuring the proportion of ALP to serumaminotransferases,where a greater proportion indicates a higher likelihood of cholestasis.[77]Typically, aminotransferase enzymes are localized withinhepatocytesand leak across the membrane upon damage.[80]However, measurement of serumaminotransferaselevels alone is not a good marker to determine cholestasis. In up to a third of patients, ALP levels may be elevated without the presence of cholestasis.[79]As such, other biomarkers should be measured to corroborate findings.
Measurement of5' nucleosidaselevels may be used to identify cholestasis in conjunction with ALP. Levels of ALP may rise within a few hours of cholestasis onset while 5' nucleosidase levels may take a few days.[81]Many labs cannot measure 5' nucleosidase and ALP levels so,GGTmay be measured in some cases.[77]Abnormal GGT elevation may be attributable to a variety of factors.[82]As such, GGT elevations lack the necessary specificity to be a useful confirmatory test for cholestasis.[77]
Importantly, conjugatedhyperbilirubinemiais present in 80% of patients with extrahepatic cholestasis and 50% of patients with intrahepatic cholestasis.[79]Given that many patients with hyperbilirubinemia may not have cholestasis, the measurement of bilirubin levels is not a good diagnostic tool for identifying cholestasis.[77]In a later stage of cholestasisaspartate transaminase(AST),alanine transaminase(ALT) andunconjugated bilirubinmay be elevated due to hepatocyte damage as a secondary effect of cholestasis.
Imaging[edit]
After determination using biomarkers, a variety of imaging studies may be used to differentiate between intrahepatic or extrahepatic cholestasis.Ultrasoundis often used to identify the location of the obstruction[83]but, is often insufficient in determining the level of biliary obstruction or its cause because it can pick up bowel gas that may interfere with readings.[77][84]CT scansare not impacted by bowel gas and may also be more suitable for overweight patients.[77]Typically, the cause of cholestasis and magnitude of obstruction is better diagnosed with CT compared to ultrasound.[85]MRI scansprovide similar information to CT scans but are more prone to interference from breathing or other bodily functions.[86]
Although CT, ultrasound, and MRI may help differentiate intrahepatic and extrahepatic cholestasis, the cause and extent of obstruction is best determined bycholangiography.[77]Potential causes of extrahepatic cholestasis include obstructions outside the wall of the lumen, those outside the duct, and obstructions found in the duct lumen.[77]Endoscopic retrograde cholangiography may be useful to visualize the extrahepatic biliary ducts.[87]In case of anatomical anomalies, or if endoscopic retrograde cholangiography is unsuccessful,percutaneous transhepatic cholangiographymay be used.[77]CT or MRI-basedcholangiographymay also be useful, particularly in cases where additional interventions are not anticipated.[77]
Histopathology[edit]
There is a significant overlap between cholestasis resulting from a hepatocellular origin and cholestasis caused by bile duct obstruction. Due to this, obstructive cholestasis can only be diagnosed after finding additional diagnostic signs that are specific to obstructive changes to the bile ducts or portal tracts.[88]In both non-obstructive and obstructive cholestasis, there is an accumulation of substances that are typically secreted in the bile, as well as degeneration ofhepatocytes.[89]The most significant feature from a histopathological perspective includes pigmentation resulting from the retention of bilirubin. Under amicroscope,the individual hepatocytes will have a brownish-greenstippledappearance within thecytoplasm,representing bile that cannot get out of the cell. Pigmentation can involve regurgitation of bile into the sinusoidal spaces caused byphagocytosisfromKupffer cells,an accumulation of bilirubin within hepatocytes, and inspissated bile in thecanaliculi.[88]Most pigmentation and canaliculi dilation occurs in the perivenular region of the hepatic lobule. In chronic cases, this may extend into the periportal area.[88]
Hepatocytenecrosisis not a significant feature of cholestasis; however, apoptosis may often occur.[90]Under the microscope,hepatocytesin the perivenular zone appear enlarged and flocculent.[88]In cases of obstructive cholestasis, bile infarcts may be produced during the degeneration and necrosis of hepatocytes.[90]Bile infarcts are marked by a large amount of pigmented tissue surrounded by a ring of necrotic hepatocytes.[88]In some cases, hepatocyte degeneration is uncommon. E.g., withAlagille syndromelimited degeneration occurs, however, there may be a small amount of apoptosis and enlarged hepatocytes.[91]
Cholestasis is often marked by cholate stasis, which are a set of changes that occur in the periportal hepatocytes.[92]Cholate stasis is more common in obstructive cholestasis compared to non-obstructive cholestasis.[93]During the cholate stasis process, hepatocytes first undergo swelling and then degeneration.[94]Under the microscope, this is evident as a lucent cell periphery and enlargedcytoplasmaround thenucleus.[93]Oftentimes,Mallory bodiesmay also be found in the periportal areas.[95]Due to the retention of bile, which containscopper,stains made for staining copper-associated protein can be used to visualize bile accumulation in the hepatocytes.[96]
Cholestatic liver cell rosettes may occur in children with chronic cholestasis.[97]Histologically, this is evident as two or more hepatocytes in a pseudotubular fashion that encircle a segment of enlarged bile canaliculi.[93]Children may also have giant hepatocytes present, which are characterized by a pigmented spongy appearance.[98]Giant cell formation is likely caused by the detergent properties ofbile saltscausing a loss of the lateral membrane and joining of hepatocytes.[99]In the case ofAlagille syndrome,hepatocyte degeneration is uncommon.[91]However, there may be a small amount of apoptosis and enlarged hepatocytes.[93]
In non-obstructive cholestasis, changes to the portal tracts are unlikely.[93]However, it may occur in some unique situations. In the case of neutrophilic pericholangitis,neutrophilssurround the portal ducts and obstruct them.[93]Neutrophilic pericholangitis has a variety of causes includingendotoxemia,Hodgkin's disease,among others.[100][101]Cholangitis lenta can also cause changes to the portal tracts.[102]This occurs during chronic cases ofsepsisand results in dilation of the bile ductules.[93]Cholangitis lenta is likely a result of a stoppage of bile secretion and bile flow through the ductules.
Back pressure created from obstructive cholestasis can cause dilation of the bile duct and biliary epithelial cell proliferation, mainly in the portal tracts.[103]Portal tractedemamay also occur as a result of bile retention, as well as periductular infiltration of neutrophils.[93]If the obstruction is left untreated, it can lead to a bacterial infection of the biliary tree.[104]Infection is mostly caused bycoliformsandenterococciand is evident from a large migration of neutrophils to the duct lumina.[93]This can result in the formation of a cholangitic abscess. With treatment, many of the histological features of cholestasis can be corrected once the obstruction is removed.[93]If the obstruction is not promptly resolved, portal tract fibrosis can result. Even with treatment, somefibrosismay remain.[93]
Management[edit]
This sectionneeds morereliable medical referencesforverificationor relies too heavily onprimary sources.(November 2021) |  |
Surgical management[edit]
In cases involving obstructive cholestasis, the primary treatment includes biliary decompression.[105]Ifbile stonesare present in thecommon bile duct,an endoscopic sphincterotomy can be conducted either with or without placing astent.[2]To do this, aduodenoscopeis placed by the endoscopist in the second portion of the duodenum. A catheter and guidewire is moved up into the common bile duct. Asphincterotomecan then enlarge theampulla of Vaterand release the stones.[106]Later, the endoscopist can place a stent in the common bile duct to soften any remaining stones and allow for bile drainage. If needed, aballoon catheteris available to remove any leftover stones. If these stones are too large with these methods, surgical removal may be needed. Patients can also request an elective cholecystectomy to prevent future cases of choledocholithiasis.[107][108]In case of narrowing of the common bile duct, a stent can be placed after dilating the constriction to resolve the obstruction.[109]
The treatment approach for patients with obstructive cholestasis resulting fromcancervaries based on whether they are a suitable candidate for surgery. In most cases, surgical intervention is the best option.[2]For patients whom complete removal of the biliary obstruction is not possible, a combination of agastric bypassandhepaticojejunostomycan be used.[110]This can reestablish bile flow into thesmall intestine,thereby bypassing the blockage. In cases where a patient is not a suitable candidate for surgery, anendoscopic stentcan be placed.[111]If this is not possible or successful, apercutaneous transhepatic cholangiogramand percutaneous biliary drainage can be used to visualize the blockage and re-establish bile flow.[112]
Medical management[edit]
A significant portion of patients with cholestasis (80%) will experiencepruritusat some point during their disease.[113][114]This is a condition that can severely decrease a patient's quality of life as it can impact sleep, concentration, work ability, and mood. Many treatments exist, but how effective each option is depends on the patient and their condition. Assessment using a scale, such as avisual analogue scaleor a 5-D itch scale will be useful to identify an appropriate treatment.[115]Possible treatment options includeantihistamines,ursodeoxycholic acid,andphenobarbital.Nalfurafine hydrochloridecan also be used to treat pruritus caused by chronic liver disease and was recently approved in Japan for this purpose.[116]
Bile acid binding resins likecholestyramineare the most common treatment. Side effects of this treatment are limited and include constipation and bloating. Other commonly used treatments includerifampin,naloxone,andsertraline.
In cholestatic liver disease, when bilirubin concentration starts to build up, a deficiency of fat soluble vitamins may also occur.[117]To manage this, doses of vitamin A, D, E, and K are recommended to retain appropriate vitamin levels.
Cholestatic liver disease can impact lipids, and possibly lead todyslipidemia,which may present a risk forcoronary artery disease.[118]Statinsandfibratesare generally used as lipid lowering therapy to treat patients with cholestatic liver disease.
For intrahepatic cholestasis in pregnant women,S-adenosylmethioninehas proven to be an effective treatment.[119]Dexamethasoneis a viable treatment in regards to the symptom of intensive itching.[120]
Research directions[edit]
Primary sclerosing cholangitis(PSC) is one of the most common cholestatic liver diseases, yet treatment options remain limited. Treatment forprimary biliary cholangitis(PBC) is often done withursodeoxycholic acid(UDCA) and with no other suitable alternative, it poses a problem for those that are not responsive to (UDCA). However, with advancing technology in the molecular biochemistry field and higher understanding of bile acid regulation, novel pharmacological treatments have been considered.
For patients withprimary biliary cholangitis,current guidelines recommend about 13–15 mg/kg ofursodeoxycholic acidas a first line treatment.[121]This drug stimulates biliary bicarbonate secretion, improves survival without having to resort to a liver transplantation, and is very well tolerated— making it an ideal treatment.[122]However, around 40% of patients with primary biliary cholangitis are not responsive to UDCA.[123]
Obeticholic acidhas been approved by the USFood and Drug Administrationfor PBC in 2016 after experiments found beneficial improvements for the liver in half of patients with inadequate response to UDCA.[124]
Primary sclerosing cholangitisis a challenging liver disease as treatment options are limited. There is still uncertainty about the efficacy ofursodeoxycholic acidfor PSC and researchers offer conflicting recommendations.[125]One study found UDCA had improved biochemical functions but did lower the rate for death or transplant-free survival.[126]
Peroxisomes receptor agonists[edit]
An important regulator in bile acid homeostasis is the alpha and delta isoforms of theperoxisome proliferator-activated receptor(PPARα,PPARδ).[127]The function of PPARα is that it promotes bile acid excretion and lowers inflammation by acting on nuclear transcription factors.[128]A well known agonist arefibratesand in the clinical trials, there was a significant biochemical response in most patients.[129]A combination therapy withbezafibrateshowed remarkable biochemical improvement, with 67% of patients normalizing theiralkaline phosphataselevels.[130]Another study of 48 patients with PBC found a combination of bezafibrate and UDCA showed a decrease of alkaline phosphatase in all patients. Further, the study found those treated had a marked relief inpruritus.[131]
However,fibratesare associated with a number of adverse effects includingarthritis,legedema,polydipsia,andmyalgias.[129]Elevations ofcreatinineandcreatine phosphokinasewere also found over a long term use.[132]
Farnesoid X-receptor agonist[edit]
A new novel treatment option is thefarnesoid X receptoris responsible for regulating bile acid homeostasis. An agonist of this nuclear hormone receptor is seen as a possible treatment as it can downregulate bile acid synthesis and reabsorption.[133]Further the farnesoid X receptor is partly responsible for lipid and glucose homeostasis, as well as pathogen recognition.[134]An agonist for the farnesoid X receptor can therefore lead to an anti-cholestatic environment to minimize the effect of toxic bile acids on the liver.[128]A candidate agonist for the farnesoid X receptor isobeticholic acidwith experiments showing it has very strong affinity.[124]A worry though is despite benefits in the biochemical pathways,prurituswas more intense and prevalent than the placebo. A titration strategy may help to mitigate pruritus, but FDA approval for obeticholic acid is currently unlikely.[135]In fact, in February 2018, the FDA gave a black box warning for OCA.[136]A recent study did find that if the drug is given with UDCA, the incidence forcirrhosisand liver transplants decreases.[137]
Another target that is being looked into is theall-transretinoic acid(ATRA), an activator for the retinoid X receptor. In vitro and animal studies found ATRA had lowered the amount of bile acid and decreased hepatic inflammation.[138]
24-norursodeoxycholic acid[edit]
A recent scientific breakthrough for cholestasis that has allowed us to evaluate a new treatment option is that a hydrophilic environment andbicarbonateproduction protects hepatocytes from bile acid.[127]The novel agent norUDCA (24-norursodeoxycholic acid) can be passively absorbed bycholangiocytes.This leads to bicarbonate production and an environment that is less toxic.[139][140]Mouse models have found promising results with norUDCA with the drug showing antiproliferative and anti-inflammatory properties. A recent clinical trial found norUDCA had significant dose-dependent reductions for ALP levels.[141]This makes norUDCA a viable possibility to look into as it clearly plays a significant role in the treatment of cholestasis.
Immunomodulatory treatments[edit]
In PBC, the liver is filled withT cellsandB cellsthat contribute to a worsening condition.[142]Therefore, some treatments are looking into targeting the antigens of these immune cells. The monoclonal antibodyrituximabtargets theCD20antigen on the B cells, and is already used in a wide array of otherrheumatologic diseases.In an open-label study, six patients that were unresponsive to UDCA had improvement in ALP levels after rituximab infusions.[143]However, the efficacy of rituximab is still uncertain, and awaits further studies and trials. PBC can also lead to higher levels ofinterleukin 12andinterleukin 23.[144]This was what motivated researches to look at the viability ofustekinumab,amonoclonal antibodytargeted against interleukin 12 and 23. An experiment found though it did not significantly improve serum ALP levels. The researchers were further even criticized for placing patients at risk by allowing them to move to advanced disease stages where immunomodulatory therapies may not even be an option.[145]
Gut microbiome[edit]
In several chronic liver diseases, thegut microbiome,which regulates both the innate and adaptive immune systems, is implicated. This can result in abnormal immunological development and an accumulation of primary bile acids. Using this information a bile-acid–intestinal-microbiota–cholestasis triangle is thought to be involved in the pathogenesis ofPBCandPSC.[146]After all, bile acids do modulate the gut microbiota; a disturbance here can result in development and progression of cholestasis. This information has prompted researchers into manipulating the microbiota viaantibioticsandprobioticsfor new treatment options. Some antibiotics examined for PSC includevancomycin,which has extensively studied and reviewed.[147]The usage of the drug is found along with a significant decrease in ALP levels, although the long term clinical benefit is unknown.[148]
As biochemistry technology becomes more advanced, promising targets have appeared, prompting numerous studies and trials to evaluate the feasibility. Fibrates, FXR agonists, and norUDCA are all innovative therapies for cholestasis.
See also[edit]
- Jaundice
- Liver function tests
- Lipoprotein-X- an abnormallow density lipoproteinfound in cholestasis
- Intrahepatic cholestasis of pregnancy
- Progressive familial intrahepatic cholestasis
- Feathery degeneration- ahistopathologicfinding associated with cholestasis
References[edit]
- ^ab"Cholestasis: Background, Pathophysiology, Epidemiology".2021-04-03.
{{cite journal}}:Cite journal requires|journal=(help) - ^abcdefghShah R, John S (2021)."Cholestatic Jaundice".StatPearls.Treasure Island (FL): StatPearls Publishing.PMID29489239.Retrieved2021-12-02.
- ^Brady P (May 2016). "Commentary on" Prospective Evaluation of the Clinical Features of Choledocholithiasis: Focus on Abdominal Pain "".Southern Medical Journal.109(5): 294–295.doi:10.14423/smj.0000000000000462.PMID27135725.
- ^Schirmer BD, Winters KL, Edlich RF (2005). "Cholelithiasis and cholecystitis".Journal of Long-Term Effects of Medical Implants.15(3): 329–338.doi:10.1615/JLongTermEffMedImplants.v15.i3.90.PMID16022643.
- ^Bergasa NV (June 2015)."The pruritus of cholestasis: facts".Hepatology.61(6): 2114.doi:10.1002/hep.27582.PMID25345776.S2CID43356558.
- ^abLangedijk JA, Beuers UH, Oude Elferink RP (2021)."Cholestasis-Associated Pruritus and Its Pruritogens".Frontiers in Medicine.8:639674.doi:10.3389/fmed.2021.639674.PMC8006388.PMID33791327.
- ^Gordon SC (September 1991). "Jaundice and cholestasis. Some common and uncommon causes".Postgraduate Medicine.90(4): 65–71.doi:10.1080/00325481.1991.11701060.PMID1891435.
- ^Fargo MV, Grogan SP, Saguil A (February 2017)."Evaluation of Jaundice in Adults".American Family Physician.95(3): 164–168.PMID28145671.
- ^Kumar D, Tandon RK (July 2002)."Fatigue in cholestatic liver disease--a perplexing symptom".Postgraduate Medical Journal.78(921): 404–407.doi:10.1136/pmj.78.921.404.PMC1742427.PMID12151655.
- ^Pearson HJ, Mosser JL, Jacks SK (November 2017). "The triad of pruritus, xanthomas, and cholestasis: Two cases and a brief review of the literature".Pediatric Dermatology.34(6): e305–e308.doi:10.1111/pde.13306.PMID29144045.S2CID13254835.
- ^Zak A, Zeman M, Slaby A, Vecka M (June 2014)."Xanthomas: clinical and pathophysiological relations".Biomedical Papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia.158(2): 181–188.doi:10.5507/bp.2014.016.PMID24781043.
- ^Parker F (1985). "Xanthomas and hyperlipidemias".Journal of the American Academy of Dermatology.13(1): 1–30.doi:10.1016/S0190-9622(85)70139-9.PMID4031142.
- ^Rods J, Benhamou JP, Blei AT, Reichen J, Rizzetto M, eds. (2007-06-18).Textbook of Hepatology.Oxford, UK: Blackwell Publishing Ltd.doi:10.1002/9780470691861.ISBN978-0-470-69186-1.
- ^Sokol RJ (December 1994). "Fat-soluble vitamins and their importance in patients with cholestatic liver diseases".Gastroenterology Clinics of North America.23(4): 673–705.doi:10.1016/s0889-8553(21)00165-5.PMID7698827.
- ^abcdeHilscher MB, Kamath PS, Eaton JE (October 2020)."Cholestatic Liver Diseases: A Primer for Generalists and Subspecialists".Mayo Clinic Proceedings.95(10): 2263–2279.doi:10.1016/j.mayocp.2020.01.015.PMID33012354.S2CID222154067.
- ^Hoerning A, Raub S, Dechêne A, Brosch MN, Kathemann S, Hoyer PF; et al. (2014)."Diversity of disorders causing neonatal cholestasis - the experience of a tertiary pediatric center in Germany".Front Pediatr.2:65.doi:10.3389/fped.2014.00065.PMC4066316.PMID25003101.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^Suriawinata AA, Thung SN (2011).Liver Pathology: An Atlas and Concise Guide.Springer.p. 47.ISBN978-1-935281-48-1.
- ^abcdCarey EJ, Lindor KD (2014).Cholestatic liver disease(Second ed.). New York, NY:Humana Press.ISBN978-1-4939-1013-7.OCLC885025528.
- ^Hussain, Nazneen; Igbinedion, Samuel O.; Diaz, Richie; Alexander, J. S.; Boktor, Moheb; Knowles, Kurt (22 October 2018)."Liver Cholestasis Secondary to Syphilis in an Immunocompetent Patient".Case Reports in Hepatology.2018:1–3.doi:10.1155/2018/8645068.PMC6217883.PMID30425865.
- ^Huang, Jiaofeng; Lin, Su; Wang, Mingfang; Wan, Bo; Zhu, Yueyong (December 2019)."Syphilitic hepatitis: a case report and review of the literature".BMC Gastroenterology.19(1): 191.doi:10.1186/s12876-019-1112-z.PMC6862847.PMID31744461.
- ^Mohi-ud-din R, Lewis JH (February 2004). "Drug- and chemical-induced cholestasis".Clinics in Liver Disease.8(1): 95–132, vii.doi:10.1016/S1089-3261(03)00124-7.PMID15062196.
- ^Padda MS, Sanchez M, Akhtar AJ, Boyer JL (April 2011)."Drug-induced cholestasis".Hepatology.53(4): 1377–1387.doi:10.1002/hep.24229.PMC3089004.PMID21480339.
- ^abDavid, Stefan; Hamilton, James P. (2010-01-01)."Drug-induced Liver Injury".US Gastroenterology & Hepatology Review.6:73–80.PMC3160634.PMID21874146.
- ^abcdefghijGijbels E, Vilas-Boas V, Deferm N, Devisscher L, Jaeschke H, Annaert P, Vinken M (May 2019)."Mechanisms and in vitro models of drug-induced cholestasis"(PDF).Archives of Toxicology.93(5): 1169–1186.doi:10.1007/s00204-019-02437-2.PMID30972450.S2CID106408884.
- ^Lisi DM (December 2016)."Drug-induced liver injury: An overview".US Pharmacist.41(12). Somerset, New: 30–4.
- ^Katarey D, Verma S (December 2016)."Drug-induced liver injury".Clinical Medicine.16(Suppl 6): s104–s109.doi:10.7861/clinmedicine.16-6-s104.PMC6329561.PMID27956449.
- ^abcdefFernández-Murga ML, Petrov PD, Conde I, Castell JV, Goméz-Lechón MJ, Jover R (October 2018). "Advances in drug-induced cholestasis: Clinical perspectives, potential mechanisms and in vitro systems".Food and Chemical Toxicology.120:196–212.doi:10.1016/j.fct.2018.07.017.PMID29990576.S2CID51612789.
- ^ab"Cholestatic Hepatitis".LiverTox: Clinical and Research Information on Drug-Induced Liver Injury.Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. 2012.PMID31689040.Retrieved2021-11-16.
- ^abcdefghijkSundaram V, Björnsson ES (October 2017)."Drug-induced cholestasis".Hepatology Communications.1(8): 726–735.doi:10.1002/hep4.1088.PMC5678916.PMID29404489.
- ^"Vanishing Bile Duct Syndrome",LiverTox: Clinical and Research Information on Drug-Induced Liver Injury,Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, 2012,PMID31689039,retrieved2021-12-05
- ^"Bland Cholestasis",LiverTox: Clinical and Research Information on Drug-Induced Liver Injury,Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, 2012,PMID31689032,retrieved2021-11-16
- ^abcdeYokoda RT, Carey EJ (October 2019). "Primary Biliary Cholangitis and Primary Sclerosing Cholangitis".The American Journal of Gastroenterology.114(10): 1593–1605.doi:10.14309/ajg.0000000000000268.PMID31169523.S2CID174811274.
- ^abcdeGulamhusein AF, Hirschfield GM (February 2020). "Primary biliary cholangitis: pathogenesis and therapeutic opportunities".Nature Reviews. Gastroenterology & Hepatology.17(2): 93–110.doi:10.1038/s41575-019-0226-7.PMID31819247.S2CID208869625.
- ^abcdefghLleo A, Leung PS, Hirschfield GM, Gershwin EM (February 2020). "The Pathogenesis of Primary Biliary Cholangitis: A Comprehensive Review".Seminars in Liver Disease.40(1): 34–48.doi:10.1055/s-0039-1697617.PMID31537031.S2CID202702083.
- ^Muratori P, Granito A, Pappas G, Muratori L, Quarneti C, De Molo C, et al. (August 2007)."Clinical and serological profile of primary biliary cirrhosis in men".QJM.100(8): 534–535.doi:10.1093/qjmed/hcm059.PMID17609225.
- ^Smyk DS, Rigopoulou EI, Pares A, Billinis C, Burroughs AK, Muratori L, et al. (2012)."Sex differences associated with primary biliary cirrhosis".Clinical & Developmental Immunology.2012:610504.doi:10.1155/2012/610504.OCLC798290161.PMC3369468.PMID22693524.
- ^Chang JC, Go S, Verhoeven AJ, Beuers U, Oude Elferink RP (April 2018)."Role of the bicarbonate-responsive soluble adenylyl cyclase in cholangiocyte apoptosis in primary biliary cholangitis; a new hypothesis".Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.1864(4 Pt B): 1232–1239.doi:10.1016/j.bbadis.2017.09.022.PMID28962898.
- ^abTanaka A (January 2021)."Current understanding of primary biliary cholangitis".Clinical and Molecular Hepatology.27(1): 1–21.doi:10.3350/cmh.2020.0028.PMC7820210.PMID33264835.
- ^abcdefghijklmnoLazaridis KN, LaRusso NF (September 2016)."Primary Sclerosing Cholangitis".The New England Journal of Medicine.375(12): 1161–1170.doi:10.1056/NEJMra1506330.PMC5553912.PMID27653566.
- ^abcdefghijklDyson JK, Beuers U, Jones DE, Lohse AW, Hudson M (June 2018). "Primary sclerosing cholangitis".Lancet.391(10139): 2547–2559.doi:10.1016/S0140-6736(18)30300-3.PMID29452711.S2CID3333034.
- ^abcdefghiLittle R, Wine E, Kamath BM, Griffiths AM, Ricciuto A (June 2020)."Gut microbiome in primary sclerosing cholangitis: A review".World Journal of Gastroenterology.26(21): 2768–2780.doi:10.3748/wjg.v26.i21.2768.PMC7284173.PMID32550753.
- ^Karlsen TH, Folseraas T, Thorburn D, Vesterhus M (December 2017)."Primary sclerosing cholangitis - a comprehensive review".Journal of Hepatology.67(6): 1298–1323.doi:10.1016/j.jhep.2017.07.022.PMID28802875.
- ^abcdefgDean, Gregory; Hanauer, Stephen; Levitsky, Josh (2020)."The Role of the Intestine in the Pathogenesis of Primary Sclerosing Cholangitis: Evidence and Therapeutic Implications".Hepatology.72(3): 1127–1138.doi:10.1002/hep.31311.ISSN0270-9139.PMID32394535.S2CID218600521.
- ^Torres, J.; Palmela, C.; Brito, H.; Bao, X.; Ruiqi, H.; Moura-Santos, P.; Silva, J. Pereira da; Oliveira, A.; Vieira, C.; Perez, K.; Itzkowitz, S. H. (2018)."The gut microbiota, bile acids and their correlation in primary sclerosing cholangitis associated with inflammatory bowel disease".United European Gastroenterology Journal.6(1): 112–122.doi:10.1177/2050640617708953.PMC5802676.PMID29435321.
- ^Sugita, Tomonori; Amano, Katsushi; Nakano, Masanori; Masubuchi, Noriko; Sugihara, Masahiro; Matsuura, Tomokazu (2015-03-03)."Analysis of the Serum Bile Acid Composition for Differential Diagnosis in Patients with Liver Disease".Gastroenterology Research and Practice.2015:e717431.doi:10.1155/2015/717431.ISSN1687-6121.PMC4363704.PMID25821461.
- ^Xie, Ai-jin; Mai, Chu-tian; Zhu, Yi-Zhun; Liu, Xian-Cheng; Xie, Ying (2021-12-15)."Bile acids as regulatory molecules and potential targets in metabolic diseases".Life Sciences.287:120152.doi:10.1016/j.lfs.2021.120152.ISSN0024-3205.PMID34793769.S2CID244281060.
- ^abcdefghijklmnopNguyen KD, Sundaram V, Ayoub WS (July 2014)."Atypical causes of cholestasis".World Journal of Gastroenterology.20(28): 9418–9426.doi:10.3748/wjg.v20.i28.9418.PMC4110573.PMID25071336.
- ^Jacquemin E (September 2012). "Progressive familial intrahepatic cholestasis".Clinics and Research in Hepatology and Gastroenterology.36(Suppl 1): S26–S35.doi:10.1016/s2210-7401(12)70018-9.PMID23141890.
- ^abDavit-Spraul A, Fabre M, Branchereau S, Baussan C, Gonzales E, Stieger B, et al. (May 2010)."ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history".Hepatology.51(5): 1645–1655.doi:10.1002/hep.23539.PMID20232290.S2CID9797502.
- ^Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E (May 2010). "The spectrum of liver diseases related to ABCB4 gene mutations: pathophysiology and clinical aspects".Seminars in Liver Disease.30(2): 134–146.doi:10.1055/s-0030-1253223.PMID20422496.S2CID260314414.
- ^Nayagam, Jeremy S.; Williamson, Catherine; Joshi, Deepak; Thompson, Richard J. (2020)."Review article: liver disease in adults with variants in the cholestasis-related genes ABCB11, ABCB4 and ATP8B1".Alimentary Pharmacology & Therapeutics.52(11–12): 1628–1639.doi:10.1111/apt.16118.ISSN1365-2036.PMID33070363.S2CID224782897.
- ^Emerick KM, Rand EB, Goldmuntz E, Krantz ID, Spinner NB, Piccoli DA (March 1999)."Features of Alagille syndrome in 92 patients: frequency and relation to prognosis".Hepatology.29(3): 822–829.doi:10.1002/hep.510290331.PMID10051485.S2CID5690401.
- ^Kamath BM, Schwarz KB, Hadzić N (January 2010)."Alagille syndrome and liver transplantation".Journal of Pediatric Gastroenterology and Nutrition.50(1): 11–15.doi:10.1097/mpg.0b013e3181c1601f.PMID19949348.S2CID22964411.
- ^Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, et al. (July 1997)."Mutations in the human Jagged1 gene are responsible for Alagille syndrome".Nature Genetics.16(3): 235–242.doi:10.1038/ng0797-235.PMID9207787.S2CID5775213.
- ^McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB (July 2006)."NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway".American Journal of Human Genetics.79(1): 169–173.doi:10.1086/505332.PMC1474136.PMID16773578.
- ^Chand N, Sanyal AJ (January 2007)."Sepsis-induced cholestasis".Hepatology.45(1): 230–241.doi:10.1002/hep.21480.PMID17187426.S2CID206643.
- ^Guglielmi FW, Regano N, Mazzuoli S, Fregnan S, Leogrande G, Guglielmi A, et al. (February 2008). "Cholestasis induced by total parenteral nutrition".Clinics in Liver Disease.12(1): 97–110, viii.doi:10.1016/j.cld.2007.11.004.PMID18242499.
- ^Cavicchi M, Beau P, Crenn P, Degott C, Messing B (April 2000). "Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure".Annals of Internal Medicine.132(7): 525–532.doi:10.7326/0003-4819-132-7-200004040-00003.PMID10744588.S2CID26657365.
- ^Jeejeebhoy KN (February 2006). "Management of short bowel syndrome: avoidance of total parenteral nutrition".Gastroenterology.130(2 Suppl 1): S60–S66.doi:10.1053/j.gastro.2005.10.065.PMID16473074.
- ^abcdefghPauli-Magnus C, Meier PJ, Stieger B (May 2010)."Genetic determinants of drug-induced cholestasis and intrahepatic cholestasis of pregnancy"(PDF).Seminars in Liver Disease.30(2): 147–159.doi:10.1055/s-0030-1253224.PMID20422497.S2CID260312828.
- ^abWood AM, Livingston EG, Hughes BL, Kuller JA (February 2018). "Intrahepatic Cholestasis of Pregnancy: A Review of Diagnosis and Management".Obstetrical & Gynecological Survey.73(2): 103–109.doi:10.1097/OGX.0000000000000524.PMID29480924.S2CID3501601.
- ^abcdefLammert F, Marschall HU, Glantz A, Matern S (December 2000). "Intrahepatic cholestasis of pregnancy: molecular pathogenesis, diagnosis and management".Journal of Hepatology.33(6): 1012–1021.doi:10.1016/s0168-8278(00)80139-7.PMID11131439.
- ^Smith DD, Rood KM (March 2020). "Intrahepatic Cholestasis of Pregnancy".Clinical Obstetrics and Gynecology.63(1): 134–151.doi:10.1097/GRF.0000000000000495.PMID31764000.S2CID208275376.
- ^Kuntz E, Kuntz HD (2008).Hepatology textbook and atlas(3rd ed.). Heidelberg: Springer. p. 237.ISBN978-3-540-76839-5.OCLC325000436.
- ^abcdefghijDeferm N, De Vocht T, Qi B, Van Brantegem P, Gijbels E, Vinken M, et al. (July 2019)."Current insights in the complexities underlying drug-induced cholestasis"(PDF).Critical Reviews in Toxicology.49(6): 520–548.doi:10.1080/10408444.2019.1635081.PMID31589080.S2CID203849209.
- ^Hagenbuch B, Dawson P (February 2004)."The sodium bile salt cotransport family SLC10"(PDF).Pflügers Archiv.447(5): 566–570.doi:10.1007/s00424-003-1130-z.PMID12851823.S2CID35115446.
- ^abcXiao J, Li Z, Song Y, Sun Y, Shi H, Chen D, Zhang Y (2021)."Molecular Pathogenesis of Intrahepatic Cholestasis of Pregnancy".Canadian Journal of Gastroenterology & Hepatology.2021:6679322.doi:10.1155/2021/6679322.PMC8181114.PMID34195157.
- ^abArias IM (2020).The liver: biology and pathobiology(6th ed.). Hoboken, NJ: John Wiley & Sons. pp. 322–323.ISBN978-1-119-43684-3.OCLC1121424716.
- ^Rao ZZ, Zhang XW, Ding YL, Yang MY (2017)."miR-148a-mediated estrogen-induced cholestasis in intrahepatic cholestasis of pregnancy: Role of PXR/MRP3".PLOS ONE.12(6): e0178702.Bibcode:2017PLoSO..1278702R.doi:10.1371/journal.pone.0178702.PMC5457162.PMID28575098.
- ^abcDixon PH, Williamson C (April 2016). "The pathophysiology of intrahepatic cholestasis of pregnancy".Clinics and Research in Hepatology and Gastroenterology.40(2): 141–153.doi:10.1016/j.clinre.2015.12.008.PMID26823041.
- ^Piechota J, Jelski W (May 2020)."Intrahepatic Cholestasis in Pregnancy: Review of the Literature".Journal of Clinical Medicine.9(5): 1361.doi:10.3390/jcm9051361.PMC7290322.PMID32384779.
- ^Sepe V, Festa C, Renga B, Carino A, Cipriani S, Finamore C, et al. (January 2016)."Insights on FXR selective modulation. Speculation on bile acid chemical space in the discovery of potent and selective agonists".Scientific Reports.6(1): 19008.Bibcode:2016NatSR...619008S.doi:10.1038/srep19008.PMC4704022.PMID26740187.
- ^Kumar A, Abbas AK, Aster JC (2015).Robbins and Cotran Pathologic Basis of Disease(9th ed.). Elsevier. pp. 821–881.ISBN978-0-323-29639-7.
- ^abcdefghijklmnHu T, Wang H (August 2021). "Hepatic Bile Acid Transporters in Drug-Induced Cholestasis".Transporters and Drug-Metabolizing Enzymes in Drug Toxicity:307–337.doi:10.1002/9781119171003.ch10.ISBN9781119171003.S2CID237848662.
- ^Li T, Chiang JY (2020-01-24)."Bile Acid Metabolism in Health and Disease".The Liver:269–285.doi:10.1002/9781119436812.ch23.ISBN9781119436829.S2CID213748863.
- ^abcAndrade RJ, Chalasani N, Björnsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. (August 2019)."Drug-induced liver injury"(PDF).Nature Reviews. Disease Primers.5(1): 58.doi:10.1038/s41572-019-0105-0.PMID31439850.S2CID201125529.
- ^abcdefghijklmnLindor KD, Talwalker JA (2008).Cholestatic liver disease.Totowa, N.J.: Humana.ISBN978-1-59745-118-5.OCLC525070586.
- ^McIntyre N (1991).Oxford textbook of clinical hepatology.Oxford University Press.ISBN0-19-261968-3.OCLC23766801.
- ^abcdCecil RL, Goldman L, Bennett JC (2000).Cecil textbook of medicine.W.B. Saunders.ISBN0-7216-7996-X.OCLC45544383.
- ^McGill MR (2016-12-15)."The past and present of serum aminotransferases and the future of liver injury biomarkers".EXCLI Journal.15:817–828.doi:10.17179/excli2016-800.PMC5318690.PMID28337112.
- ^Hill PG, Sammons HG (October 1967). "An assessment of 5'-nucleotidase as a liver-function test".The Quarterly Journal of Medicine.36(144): 457–468.doi:10.1093/oxfordjournals.qjmed.a067123.PMID6077226.
- ^Gowda S, Desai PB, Hull VV, Math AA, Vernekar SN, Kulkarni SS (November 2009)."A review on laboratory liver function tests".The Pan African Medical Journal.3:17.PMC2984286.PMID21532726.
- ^Pérez Fernández T, López Serrano P, Tomás E, Gutiérrez ML, Lledó JL, Cacho G, et al. (January 2004)."Diagnostic and therapeutic approach to cholestatic liver disease".Revista Espanola de Enfermedades Digestivas.96(1): 60–73.doi:10.4321/S1130-01082004000100008.PMID14971998.
- ^Andrzejewska M, Grzymisławski M (2018)."The role of intestinal ultrasound in diagnostics of bowel diseases".Przeglad Gastroenterologiczny.13(1): 1–5.doi:10.5114/pg.2018.74554.PMC5894446.PMID29657604.
- ^Reddy SI, Grace ND (February 2002). "Liver imaging. A hepatologist's perspective".Clinics in Liver Disease.6(1): 297–310, ix.doi:10.1016/s1089-3261(03)00077-1.PMID11933595.
- ^Ladd ME, Bachert P, Meyerspeer M, Moser E, Nagel AM, Norris DG, et al. (December 2018)."Pros and cons of ultra-high-field MRI/MRS for human application".Progress in Nuclear Magnetic Resonance Spectroscopy.109:1–50.doi:10.1016/j.pnmrs.2018.06.001.PMID30527132.S2CID54474265.
- ^Singla S, Piraka C (December 2014)."Endoscopic retrograde cholangiopancreatography".Clinical Liver Disease.4(6): 133–137.doi:10.1002/cld.441.PMC6448759.PMID30992940.
- ^abcdeCrawford JM (2004). "Pathology of Cholestasis".Molecular Pathogenesis of Cholestasis.Boston, MA: Springer US. pp. 149–169.doi:10.1007/978-1-4419-9034-1_12(inactive 2024-06-22).ISBN978-1-4613-4767-5.
{{cite book}}:CS1 maint: DOI inactive as of June 2024 (link) - ^Desmet VJ (1995)."Histopathology of cholestasis".Verhandlungen der Deutschen Gesellschaft für Pathologie.79:233–240.PMID8600686.
- ^abWoolbright BL, Jaeschke H (November 2019)."Inflammation and Cell Death During Cholestasis: The Evolving Role of Bile Acids".Gene Expression.19(3): 215–228.doi:10.3727/105221619x15614873062730(inactive 2024-06-22).PMC6827039.PMID31253204.
{{cite journal}}:CS1 maint: DOI inactive as of June 2024 (link) - ^abAlagille D, Odièvre M, Gautier M, Dommergues JP (January 1975). "Hepatic ductular hypoplasia associated with characteristic facies, vertebral malformations, retarded physical, mental, and sexual development, and cardiac murmur".The Journal of Pediatrics.86(1): 63–71.doi:10.1016/s0022-3476(75)80706-2.PMID803282.
- ^Assy N, Jacob G, Spira G, Edoute Y (June 1999)."Diagnostic approach to patients with cholestatic jaundice".World Journal of Gastroenterology.5(3): 252–262.doi:10.3748/wjg.v5.i3.252.PMC4688481.PMID11819442.
- ^abcdefghijkLi MK, Crawford JM (February 2004). "The pathology of cholestasis".Seminars in Liver Disease.24(1): 21–42.doi:10.1055/s-2004-823099.PMID15085484.S2CID260319594.
- ^Kleiner DE (June 2017)."Drug-induced Liver Injury: The Hepatic Pathologist's Approach".Gastroenterology Clinics of North America.46(2): 273–296.doi:10.1016/j.gtc.2017.01.004.PMC5434713.PMID28506365.
- ^Kurtovic E, Limaiem F (2021).Mallory Bodies.Treasure Island (FL): StatPearls Publishing.PMID31424884.Retrieved2021-12-02.
{{cite book}}:|work=ignored (help) - ^Goldfischer S, Popper H, Sternlieb I (June 1980)."The significance of variations in the distribution of copper in liver disease".The American Journal of Pathology.99(3): 715–730.PMC1903690.PMID7386600.
- ^Nagore N, Howe S, Boxer L, Scheuer PJ (February 1989). "Liver cell rosettes: structural differences in cholestasis and hepatitis".Liver.9(1): 43–51.doi:10.1111/j.1600-0676.1989.tb00377.x.PMID2466188.
- ^Morotti RA, Jain D (June 2013). "Pediatric Cholestatic Disorders: Approach to Pathologic Diagnosis".Surgical Pathology Clinics.6(2): 205–225.doi:10.1016/j.path.2013.03.001.PMID26838972.
- ^Lewis J (March 2018)."Histopathology of granulomatous liver disease".Clinical Liver Disease.11(3): 77–80.doi:10.1002/cld.695.PMC6385944.PMID30992794.
- ^Crawford JM (1997). "Cellular and molecular biology of the inflamed liver".Current Opinion in Gastroenterology.13(3): 175–185.doi:10.1097/00001574-199705000-00002.ISSN0267-1379.
- ^Crawford JM, Boyer JL (July 1998)."Clinicopathology conferences: inflammation-induced cholestasis".Hepatology.28(1): 253–260.doi:10.1002/hep.510280133.PMID9657120.S2CID43255288.
- ^Lefkowitch JH (January 1982). "Bile ductular cholestasis: an ominous histopathologic sign related to sepsis and" cholangitis lenta "".Human Pathology.13(1): 19–24.doi:10.1016/s0046-8177(82)80134-2.PMID7076191.
- ^Tavoloni N, Schaffner F (1985)."The intrahepatic biliary epithelium in the guinea pig: is hepatic artery blood flow essential in maintaining its function and structure?".Hepatology.5(4): 666–672.doi:10.1002/hep.1840050424.PMID4018739.S2CID11216886.
- ^Tănăsescu C (March 2004)."Correlation between cholestasis and infection".Romanian Journal of Gastroenterology.13(1): 23–27.PMID15054522.
- ^Wang L, Yu WF (March 2014). "Obstructive jaundice and perioperative management".Acta Anaesthesiologica Taiwanica.52(1): 22–29.doi:10.1016/j.aat.2014.03.002.PMID24999215.
- ^de Clemente Junior CC, Bernardo WM, Franzini TP, Luz GO, Dos Santos ME, Cohen JM, et al. (August 2018)."Comparison between endoscopic sphincterotomyvsendoscopic sphincterotomy associated with balloon dilation for removal of bile duct stones: A systematic review and meta-analysis based on randomized controlled trials ".World Journal of Gastrointestinal Endoscopy.10(8): 130–144.doi:10.4253/wjge.v10.i8.130.PMC6107471.PMID30147845.
- ^Parikh MP, Gupta NM, Thota PN, Lopez R, Sanaka MR (April 2018). "Temporal trends in utilization and outcomes of endoscopic retrograde cholangiopancreatography in acute cholangitis due to choledocholithiasis from 1998 to 2012".Surgical Endoscopy.32(4): 1740–1748.doi:10.1007/s00464-017-5856-7.PMID28917018.S2CID39106004.
- ^Benites Goñi HE, Palacios Salas FV, Asencios Cusihuallpa JL, Aguilar Morocco R, Segovia Valle NS (April 2017)."[Performance of ASGE predictive criteria in diagnosis of choledocholithiasis in the Edgardo Rebagliati Martins Hospital]".Revista de Gastroenterologia del Peru.37(2): 111–119.PMID28731990.
- ^Ferm S, Fisher C, Hassam A, Rubin M, Kim SH, Hussain SA (2018)."Primary Endoscopic Closure of Duodenal Perforation Secondary to Biliary Stent Migration: A Case Report and Review of the Literature".Journal of Investigative Medicine High Impact Case Reports.6:2324709618792031.doi:10.1177/2324709618792031.PMC6088461.PMID30116760.
- ^Imam MH, Tejaswi S, Quraishi MN, Tabibian JH (2016)."Advances in Biliary Tract Disorders: Novel Biomarkers, Pharmacotherapies, Endoscopic Techniques, and Surgical Management".Gastroenterology Research and Practice.2016:1583486.doi:10.1155/2016/1583486.PMC5203896.PMID28074091.
- ^Kim JH (December 2011)."Endoscopic stent placement in the palliation of malignant biliary obstruction".Clinical Endoscopy.44(2): 76–86.doi:10.5946/ce.2011.44.2.76.PMC3363064.PMID22741117.
- ^Voegeli DR, Crummy AB, Weese JL (August 1985). "Percutaneous transhepatic cholangiography, drainage, and biopsy in patients with malignant biliary obstruction. An alternative to surgery".American Journal of Surgery.150(2): 243–247.doi:10.1016/0002-9610(85)90129-1.PMID2411158.
- ^Jin XY, Khan TM (September 2016)."Quality of life among patients suffering from cholestatic liver disease-induced pruritus: A systematic review".Journal of the Formosan Medical Association = Taiwan Yi Zhi.115(9): 689–702.doi:10.1016/j.jfma.2016.05.006.PMID27431691.
- ^Pedersen MR, Mayo MJ (March 2020)."Managing the Symptoms and Complications of Cholestasis".Clinical Liver Disease.15(3): 120–124.doi:10.1002/cld.901.PMC7128033.PMID32257123.
- ^Elman S, Hynan LS, Gabriel V, Mayo MJ (March 2010)."The 5-D itch scale: a new measure of pruritus".The British Journal of Dermatology.162(3): 587–593.doi:10.1111/j.1365-2133.2009.09586.x.PMC2875190.PMID19995367.
- ^Kamimura K, Yokoo T, Kamimura H, Sakamaki A, Abe S, Tsuchiya A, et al. (2017-06-12)."Long-term efficacy and safety of nalfurafine hydrochloride on pruritus in chronic liver disease patients: Patient-reported outcome based analyses".PLOS ONE.12(6): e0178991.Bibcode:2017PLoSO..1278991K.doi:10.1371/journal.pone.0178991.PMC5467861.PMID28604788.
- ^Kaplan MM, Elta GH, Furie B, Sadowski JA, Russell RM (September 1988)."Fat-soluble vitamin nutriture in primary biliary cirrhosis".Gastroenterology.95(3): 787–792.doi:10.1016/s0016-5085(88)80029-5.PMID3396823.
- ^Wang C, Zhao P, Liu W (2014)."Risk of incident coronary artery disease in patients with primary biliary cirrhosis".International Journal of Clinical and Experimental Medicine.7(9): 2921–2924.PMC4211810.PMID25356160.
- ^Frezza M, Surrenti C, Manzillo G, Fiaccadori F, Bortolini M, Di Padova C (July 1990)."Oral S-adenosylmethionine in the symptomatic treatment of intrahepatic cholestasis. A double-blind, placebo-controlled study".Gastroenterology.99(1): 211–215.doi:10.1016/0016-5085(90)91250-A.PMID2188871.
- ^Hirvioja ML, Tuimala R, Vuori J (February 1992). "The treatment of intrahepatic cholestasis of pregnancy by dexamethasone".British Journal of Obstetrics and Gynaecology.99(2): 109–111.doi:10.1111/j.1471-0528.1992.tb14465.x.PMID1554659.S2CID2391542.
- ^Floreani A, Mangini C (January 2018). "Primary biliary cholangitis: Old and novel therapy".European Journal of Internal Medicine.47:1–5.doi:10.1016/j.ejim.2017.06.020.PMID28669591.
- ^Corpechot C, Abenavoli L, Rabahi N, Chrétien Y, Andréani T, Johanet C, et al. (September 2008). "Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis".Hepatology.48(3): 871–877.doi:10.1002/hep.22428.PMID18752324.S2CID40564903.
- ^Parés A, Caballería L, Rodés J (March 2006)."Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid".Gastroenterology.130(3): 715–720.doi:10.1053/j.gastro.2005.12.029.PMID16530513.
- ^abNevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, et al. (August 2016)."A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis".The New England Journal of Medicine.375(7): 631–643.doi:10.1056/NEJMoa1509840.PMID27532829.S2CID205099018.
- ^Hirschfield GM, Beuers U, Corpechot C, Invernizzi P, Jones D, Marzioni M, Schramm C (July 2017)."EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis"(PDF).Journal of Hepatology.67(1): 145–172.doi:10.1016/j.jhep.2017.03.022.PMID28427765.
- ^Lindor KD (March 1997)."Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group".The New England Journal of Medicine.336(10): 691–695.doi:10.1056/NEJM199703063361003.PMID9041099.
- ^abBeuers U, Trauner M, Jansen P, Poupon R (April 2015)."New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond".Journal of Hepatology.62(1 Suppl): S25–S37.doi:10.1016/j.jhep.2015.02.023.PMID25920087.
- ^abde Vries E, Beuers U (January 2017)."Management of cholestatic disease in 2017".Liver International.37(Suppl 1): 123–129.doi:10.1111/liv.13306.PMID28052628.S2CID19312830.
- ^abLevy C, Peter JA, Nelson DR, Keach J, Petz J, Cabrera R, et al. (January 2011)."Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid".Alimentary Pharmacology & Therapeutics.33(2): 235–242.doi:10.1111/j.1365-2036.2010.04512.x.PMID21083674.S2CID5243731.
- ^Corpechot C, Chazouillères O, Rousseau A, Guyader D, Habersetzer F, Mathurin P, et al. (2017)."A 2-year multicenter, double-blind, randomized, placebo-controlled study of bezafibrate for the treatment of primary biliary cholangitis in patients with inadequate biochemical response to ursodeoxycholic acid therapy (Bezurso)".Journal of Hepatology.1(S)(66): S89.doi:10.1016/S0168-8278(17)30442-7.ISSN0168-8278.
- ^Reig A, Sesé P, Parés A (January 2018). "Effects of Bezafibrate on Outcome and Pruritus in Primary Biliary Cholangitis With Suboptimal Ursodeoxycholic Acid Response".The American Journal of Gastroenterology.113(1): 49–55.doi:10.1038/ajg.2017.287.PMID29016567.S2CID205105216.
- ^Iwasaki S, Ohira H, Nishiguchi S, Zeniya M, Kaneko S, Onji M, et al. (June 2008). "The efficacy of ursodeoxycholic acid and bezafibrate combination therapy for primary biliary cirrhosis: A prospective, multicenter study".Hepatology Research.38(6): 557–564.doi:10.1111/j.1872-034X.2007.00305.x.PMID18452482.S2CID36861916.
- ^Trauner M, Fuchs CD, Halilbasic E, Paumgartner G (April 2017)."New therapeutic concepts in bile acid transport and signaling for management of cholestasis".Hepatology.65(4): 1393–1404.doi:10.1002/hep.28991.PMID27997980.S2CID205903643.
- ^Ding L, Yang L, Wang Z, Huang W (March 2015)."Bile acid nuclear receptor FXR and digestive system diseases".Acta Pharmaceutica Sinica B.5(2): 135–144.doi:10.1016/j.apsb.2015.01.004.PMC4629217.PMID26579439.
- ^Kowdley KV, Luketic V, Chapman R, Hirschfield GM, Poupon R, Schramm C, et al. (May 2018)."A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis".Hepatology.67(5): 1890–1902.doi:10.1002/hep.29569.PMC5947631.PMID29023915.
- ^Eslam M, Alvani R, Shiha G (December 2019). "Obeticholic acid: towards first approval for NASH".Lancet.394(10215): 2131–2133.doi:10.1016/S0140-6736(19)32963-0.PMID31813639.S2CID208652249.
- ^Samur S, Klebanoff M, Banken R, Pratt DS, Chapman R, Ollendorf DA, et al. (March 2017)."Long-term clinical impact and cost-effectiveness of obeticholic acid for the treatment of primary biliary cholangitis".Hepatology.65(3): 920–928.doi:10.1002/hep.28932.PMID27906472.S2CID20776738.
- ^Assis DN, Abdelghany O, Cai SY, Gossard AA, Eaton JE, Keach JC, et al. (February 2017)."Combination Therapy of All-Trans Retinoic Acid With Ursodeoxycholic Acid in Patients With Primary Sclerosing Cholangitis: A Human Pilot Study".Journal of Clinical Gastroenterology.51(2): e11–e16.doi:10.1097/MCG.0000000000000591.PMC5218875.PMID27428727.
- ^Trauner M, Fickert P, Halilbasic E, Moustafa T (2008). "Lessons from the toxic bile concept for the pathogenesis and treatment of cholestatic liver diseases".Wiener Medizinische Wochenschrift.158(19–20): 542–548.doi:10.1007/s10354-008-0592-1.PMID18998069.S2CID25907527.
- ^Dumont M, Erlinger S, Uchman S (July 1980)."Hypercholeresis induced by ursodeoxycholic acid and 7-ketolithocholic acid in the rat: possible role of bicarbonate transport".Gastroenterology.79(1): 82–89.doi:10.1016/0016-5085(80)90078-5.PMID7380227.
- ^Fickert P, Hirschfield GM, Denk G, Marschall HU, Altorjay I, Färkkilä M, et al. (September 2017)."norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis".Journal of Hepatology.67(3): 549–558.doi:10.1016/j.jhep.2017.05.009.hdl:2437/248251.PMID28529147.
- ^Van de Water J, Ansari A, Prindiville T, Coppel RL, Ricalton N, Kotzin BL, et al. (February 1995)."Heterogeneity of autoreactive T cell clones specific for the E2 component of the pyruvate dehydrogenase complex in primary biliary cirrhosis".The Journal of Experimental Medicine.181(2): 723–733.doi:10.1084/jem.181.2.723.PMC2191887.PMID7836925.
- ^Tsuda M, Moritoki Y, Lian ZX, Zhang W, Yoshida K, Wakabayashi K, et al. (February 2012)."Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid".Hepatology.55(2): 512–521.doi:10.1002/hep.24748.PMID22006563.S2CID39002609.
- ^Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Lu Y, et al. (June 2009)."Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants".The New England Journal of Medicine.360(24): 2544–2555.doi:10.1056/NEJMoa0810440.PMC2857316.PMID19458352.
- ^Qian C, Jiang T, Zhang W, Ren C, Wang Q, Qin Q, et al. (October 2013). "Increased IL-23 and IL-17 expression by peripheral blood cells of patients with primary biliary cirrhosis".Cytokine.64(1): 172–180.doi:10.1016/j.cyto.2013.07.005.PMID23910013.
- ^Woodhouse CA, Patel VC, Singanayagam A, Shawcross DL (January 2018)."Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease".Alimentary Pharmacology & Therapeutics.47(2): 192–202.doi:10.1111/apt.14397.PMID29083037.S2CID36556605.
- ^Damman JL, Rodriguez EA, Ali AH, Buness CW, Cox KL, Carey EJ, Lindor KD (April 2018)."Review article: the evidence that vancomycin is a therapeutic option for primary sclerosing cholangitis".Alimentary Pharmacology & Therapeutics.47(7): 886–895.doi:10.1111/apt.14540.PMID29411404.S2CID3752599.
- ^Tabibian JH, Weeding E, Jorgensen RA, Petz JL, Keach JC, Talwalkar JA, Lindor KD (March 2013)."Randomised clinical trial: vancomycin or metronidazole in patients with primary sclerosing cholangitis - a pilot study".Alimentary Pharmacology & Therapeutics.37(6): 604–612.doi:10.1111/apt.12232.PMID23384404.S2CID22419224.
