From Wikipedia, the free encyclopedia
Chemical compound
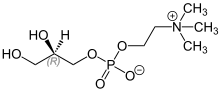
L -α-Glycerophosphorylcholinealpha-GPC ,choline alfoscerate ,sn -glycero-3-phosphocholinecholine compound found in the brain. It is also aparasympathomimetic acetylcholine precursor[1] Alzheimer's disease [2] dementias .[3]
Alpha-GPC rapidly deliverscholine to the brain across theblood–brain barrier and is a biosynthetic precursor ofacetylcholine .[2] FDA determined that intake of no more than 196.2 mg/person/day is consideredgenerally recognized as safe (GRAS).[4]
Industrially, alpha-GPC is produced by the chemical or enzymatic deacylation ofphosphatidylcholine enriched soyaphospholipids followed bychromatographic purification .Alpha-GPC may also be derived in small amounts from highly purified soylecithin as well as from purified sunflower lecithin.[5] [6]
Alpha-GPC metabolizes totrimethylamine n-oxide in the gastrointestinal tract, which has implications for cardiovascular health. In one study, risk of stroke over a ten-year period was increased by about 40% in users of alpha-GPC.[7]
^ De Jesus Moreno Moreno M (January 2003). "Cognitive improvement in mild to moderate Alzheimer's dementia after treatment with the acetylcholine precursor choline alfoscerate: a multicenter, double-blind, randomized, placebo-controlled trial".Clinical Therapeutics .25 (1): 178–93.doi :10.1016/S0149-2918(03)90023-3 .PMID 12637119 . ^a b Parnetti L, Mignini F, Tomassoni D, Traini E, Amenta F (June 2007). "Cholinergic precursors in the treatment of cognitive impairment of vascular origin: ineffective approaches or need for re-evaluation?".Journal of the Neurological Sciences .257 (1–2): 264–9.doi :10.1016/j.jns.2007.01.043 .PMID 17331541 .S2CID 34661218 . ^ Doggrell SA, Evans S (October 2003). "Treatment of dementia with neurotransmission modulation".Expert Opinion on Investigational Drugs .12 (10): 1633–54.doi :10.1517/13543784.12.10.1633 .PMID 14519085 .S2CID 46175609 . ^ "Generally Recognized as Safe (GRAS) Determination for the Use of AlphaSize® Alpha-Glycerylphosphoryl Choline" (PDF) .United States Food and Drug Administration. 25 January 2012. Archived fromthe original (PDF) on 24 December 2013.^ Traini E, Bramanti V, Amenta F (December 2013). "Choline alphoscerate (alpha-glyceryl-phosphoryl-choline) an old choline- containing phospholipid with a still interesting profile as cognition enhancing agent".Current Alzheimer Research .10 (10): 1070–9.doi :10.2174/15672050113106660173 .PMID 24156263 . ^ Scapicchio PL (July 2013). "Revisiting choline alphoscerate profile: a new, perspective, role in dementia?".The International Journal of Neuroscience .123 (7): 444–9.doi :10.3109/00207454.2013.765870 .PMID 23387341 . ^ Lee G, Choi S, Chang J, Choi D, Son JS, Kim K, et al. (November 2021)."Association of L-α Glycerylphosphorylcholine With Subsequent Stroke Risk After 10 Years" .JAMA Network Open .4 (11): e2136008.doi :10.1001/jamanetworkopen.2021.36008 .PMC 8613599 PMID 34817582 .
mAChRs Tooltip Muscarinic acetylcholine receptors
Agonists Antagonists
3-Quinuclidinyl benzilate 4-DAMP Aclidinium bromide (+formoterol )Abediterol AF-DX 250 AF-DX 384 Ambutonium bromide Anisodamine Anisodine Antihistamines (first-generation) (e.g.,brompheniramine ,buclizine ,captodiame ,chlorphenamine (chlorpheniramine) ,cinnarizine ,clemastine ,cyproheptadine ,dimenhydrinate ,dimetindene ,diphenhydramine ,doxylamine ,meclizine ,mequitazine ,perlapine ,phenindamine ,pheniramine ,phenyltoloxamine ,promethazine ,propiomazine ,triprolidine )AQ-RA 741 Atropine Atropine methonitrate Atypical antipsychotics (e.g.,clozapine ,fluperlapine ,olanzapine (+fluoxetine ),rilapine ,quetiapine ,tenilapine ,zotepine )Benactyzine Benzatropine (benztropine) Benzilone Benzilylcholine mustard Benzydamine Bevonium BIBN 99 Biperiden Bornaprine Camylofin CAR-226,086 CAR-301,060 CAR-302,196 CAR-302,282 CAR-302,368 CAR-302,537 CAR-302,668 Caramiphen Cimetropium bromide Clidinium bromide Cloperastine CS-27349 Cyclobenzaprine Cyclopentolate Darifenacin DAU-5884 Desfesoterodine Dexetimide DIBD Dicycloverine (dicyclomine) Dihexyverine Difemerine Diphemanil metilsulfate Ditran Drofenine EA-3167 EA-3443 EA-3580 EA-3834 Emepronium bromide Etanautine Etybenzatropine (ethybenztropine) Fenpiverinium Fentonium bromide Fesoterodine Flavoxate Glycopyrronium bromide (+beclometasone/formoterol ,+indacaterol ,+neostigmine )Hexahydrodifenidol Hexahydrosiladifenidol Hexbutinol Hexocyclium Himbacine HL-031,120 Homatropine Imidafenacin Ipratropium bromide (+salbutamol )Isopropamide J-104,129 Hyoscyamine Mamba toxin 3 Mamba toxin 7 Mazaticol Mebeverine Meladrazine Mepenzolate Methantheline Methoctramine Methylatropine Methylhomatropine Methylscopolamine Metixene Muscarinic toxin 7 N-Ethyl-3-piperidyl benzilate N-Methyl-3-piperidyl benzilate Nefopam Octatropine methylbromide (anisotropine methylbromide) Orphenadrine Otenzepad (AF-DX 116) Otilonium bromide Oxapium iodide Oxitropium bromide Oxybutynin Oxyphencyclimine Oxyphenonium bromide PBID PD-102,807 PD-0298029 Penthienate Pethidine pFHHSiD Phenglutarimide Phenyltoloxamine Pipenzolate bromide Piperidolate Pirenzepine Piroheptine Pizotifen Poldine Pridinol Prifinium bromide Procyclidine Profenamine (ethopropazine) Propantheline bromide Propiverine Quinidine 3-Quinuclidinyl thiochromane-4-carboxylate Revefenacin Rociverine RU-47,213 SCH-57,790 SCH-72,788 SCH-217,443 Scopolamine (hyoscine) Scopolamine butylbromide (hyoscine butylbromide) Silahexacyclium Sofpironium bromide Solifenacin SSRIs Tooltip Selective serotonin reuptake inhibitors (e.g.,femoxetine ,paroxetine )Telenzepine Terodiline Tetracyclic antidepressants (e.g.,amoxapine ,maprotiline ,mianserin ,mirtazapine )Tiemonium iodide Timepidium bromide Tiotropium bromide Tiquizium bromide Tofenacin Tolterodine Tricyclic antidepressants (e.g.,amitriptyline (+perphenazine ),amitriptylinoxide ,butriptyline ,cidoxepin ,clomipramine ,desipramine ,desmethyldesipramine ,dibenzepin ,dosulepin (dothiepin) ,doxepin ,imipramine ,lofepramine ,nitroxazepine ,northiaden (desmethyldosulepin) ,nortriptyline ,protriptyline ,quinupramine ,trimipramine )Tridihexethyl Trihexyphenidyl Trimebutine Tripitamine (tripitramine) Tropacine Tropatepine Tropicamide Trospium chloride Typical antipsychotics (e.g.,chlorpromazine ,chlorprothixene ,cyamemazine (cyamepromazine) ,loxapine ,mesoridazine ,thioridazine )Umeclidinium bromide (+vilanterol )WIN-2299 Xanomeline Zamifenacin
Precursors (andprodrugs )
nAChRs Tooltip Nicotinic acetylcholine receptors
Agonists PAMs Tooltip positive allosteric modulators )
5-HIAA 6-Chloronicotine A-84,543 A-366,833 A-582,941 A-867,744 ABT-202 ABT-418 ABT-560 ABT-894 Acetylcholine Altinicline Anabasine Anatabine Anatoxin-a AR-R17779 Bephenium hydroxynaphthoate Butinoline Butyrylcholine Carbachol Choline Cotinine Cytisine Decamethonium Desformylflustrabromine Dianicline Dimethylphenylpiperazinium Epibatidine Epiboxidine Ethanol (alcohol) Ethoxysebacylcholine EVP-4473 EVP-6124 Galantamine GTS-21 Ispronicline Ivermectin JNJ-39393406 Levamisole Lobeline MEM-63,908 (RG-3487) Morantel Nicotine (tobacco )NS-1738 PHA-543,613 PHA-709,829 PNU-120,596 PNU-282,987 Pozanicline Pyrantel Rivanicline RJR-2429 Sazetidine A SB-206553 Sebacylcholine SIB-1508Y SIB-1553A SSR-180,711 Suberyldicholine Suxamethonium (succinylcholine) Suxethonium (succinyldicholine) TC-1698 TC-1734 TC-1827 TC-2216 TC-5214 TC-5619 TC-6683 Tebanicline Tribendimidine Tropisetron UB-165 Varenicline WAY-317,538 XY-4083 Antagonists NAMs Tooltip negative allosteric modulators )
Precursors (andprodrugs )