Condensation polymer
| Polymer science |
|---|
 |

Inpolymer chemistry,condensation polymersare any kind ofpolymerswhose process ofpolymerizationinvolves acondensation reaction(i.e. a small molecule, such aswaterormethanol,is produced as a byproduct). Naturalproteinsas well as some common plastics such asnylonandPETEare formed in this way. Condensation polymers are formed by polycondensation, when the polymer is formed by condensation reactions between species of alldegrees of polymerization,or by condensativechain polymerization,when the polymer is formed by sequential addition of monomers to anactive sitein achain reaction.The main alternative forms of polymerization are chain polymerization andpolyaddition,both of which giveaddition polymers.
Polycondensation A polymerization in which the growth of polymer chains proceeds by condensation reactions between molecules of all degrees of polymerization. Notes: 1. The growth steps are expressed by: Px+Py→Px+y+L {x}∈{1,2,…∞};{y}∈{1,2,…∞}
where Pxand Pydenote chains of degrees of polymerization x and y, respectively, and L a low-molar-mass by-product. 2. The earlier term 'polycondensation' was synonymous with 'condensation polymerization'. The current definitions of polycondensation and condensative chain polymerization were both embraced by the earlier term 'polycondensation'.[1]
Condensation polymerization is a form ofstep-growth polymerization.Linear polymers are produced frombifunctionalmonomers, i.e. compounds with two reactiveend-groups.Common condensation polymers includepolyesters,polyamidessuch asnylon,polyacetals,andproteins.[2][3]
Polyamides[edit]
One important class of condensation polymers arepolyamides.[4]They arise from the reaction ofcarboxylic acidand an amine. Examples includenylonsandproteins.When prepared from amino-carboxylic acids, e.g. amino acids, the stoichiometry of the polymerization includes co-formation of water:
- n H2N-X-CO2H → [HN-X-C(O)]n+ (n-1) H2O
When prepared fromdiaminesanddicarboxylic acids,e.g. the production ofnylon 66,the polymerization produces two molecules of water per repeat unit:
- n H2N-X-NH2+ n HO2C-Y-CO2H → [HN-X-NHC(O)-Y-C(O)]n+ (2n-1) H2O

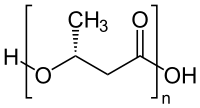
General chemical structure of one type of condensation polymer
Polyesters[edit]
Another important class of condensation polymers arepolyesters.[5]They arise from the reaction of acarboxylic acidand an alcohol. An example ispolyethyleneterephthalate,the common plastic PETE (recycling #1 in the USA):
- n HO-X-OH + n HO2C-Y-CO2H → [O-X-O2C-Y-C(O)]n+ (2n-1) H2O
Safety and environmental considerations[edit]
Condensation polymers tend to be more biodegradable thanaddition polymers.The peptide or ester bonds between monomers can be hydrolysed, especially in the presence of catalysts or bacterialenzymes.[6]
See also[edit]
References[edit]
- ^Jenkins, A. D.; Kratochvíl, P.; Stepto, R. F. T.; Suter, U. W. (1996-01-01)."Glossary of basic terms in polymer science (IUPAC Recommendations 1996)".Pure and Applied Chemistry.68(12): 2287–2311.doi:10.1351/pac199668122287.ISSN0033-4545.S2CID98774337.
- ^Introduction to Polymers1987 R.J. Young Chapman & HallISBN0-412-22170-5
- ^D. Margerison; G. C. East; J. E. Spice (1967).An Introduction to Polymer Chemistry.Pergamon Press.ISBN978-0-08-011891-8.
- ^B. Herzog; M. I. Kohan; S. A. Mestemacher; R. U. Pagilagan; K. Redmond (2013). "Polyamides".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a21_179.pub3.ISBN978-3-527-30673-2.S2CID241272519.
- ^Horst Köpnick; Manfred Schmidt; Wilhelm Brügging; Jörn Rüter; Walter Kaminsky (2002). "Polyesters".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a21_227.ISBN3-527-30673-0.
- ^Wei, Ren; Zimmermann, Wolfgang (November 2017)."Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: how far are we?".Microbial Biotechnology.10(6): 1308–1322.doi:10.1111/1751-7915.12710.PMC5658625.PMID28371373.
