Copper
 | |||||||||||||||||||||||||||||||||
| Copper | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | Red-orange metallic luster | ||||||||||||||||||||||||||||||||
| Standard atomic weightAr°(Cu) | |||||||||||||||||||||||||||||||||
| Copper in theperiodic table | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Atomic number(Z) | 29 | ||||||||||||||||||||||||||||||||
| Group | group 11 | ||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d104s1 | ||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 1 | ||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||
| PhaseatSTP | solid | ||||||||||||||||||||||||||||||||
| Melting point | 1357.77K(1084.62 °C, 1984.32 °F) | ||||||||||||||||||||||||||||||||
| Boiling point | 2835 K (2562 °C, 4643 °F) | ||||||||||||||||||||||||||||||||
| Density(at 20° C) | 8.935 g/cm3 [3] | ||||||||||||||||||||||||||||||||
| when liquid (atm.p.) | 8.02 g/cm3 | ||||||||||||||||||||||||||||||||
| Heat of fusion | 13.26kJ/mol | ||||||||||||||||||||||||||||||||
| Heat of vaporization | 300.4 kJ/mol | ||||||||||||||||||||||||||||||||
| Molar heat capacity | 24.440 J/(mol·K) | ||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||
| Oxidation states | −2, 0,[4]+1,+2,+3, +4 (a mildlybasicoxide) | ||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.90 | ||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 128pm | ||||||||||||||||||||||||||||||||
| Covalent radius | 132±4 pm | ||||||||||||||||||||||||||||||||
| Van der Waals radius | 140 pm | ||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic(fcc) (cF4) | ||||||||||||||||||||||||||||||||
| Lattice constant | a= 361.50 pm (at 20 °C)[3] | ||||||||||||||||||||||||||||||||
| Thermal expansion | 16.64×10−6/K (at 20 °C)[3] | ||||||||||||||||||||||||||||||||
| Thermal conductivity | 401 W/(m⋅K) | ||||||||||||||||||||||||||||||||
| Electrical resistivity | 16.78 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[5] | ||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −5.46×10−6cm3/mol[6] | ||||||||||||||||||||||||||||||||
| Young's modulus | 110–128 GPa | ||||||||||||||||||||||||||||||||
| Shear modulus | 48 GPa | ||||||||||||||||||||||||||||||||
| Bulk modulus | 140 GPa | ||||||||||||||||||||||||||||||||
| Speed of soundthin rod | (annealed) 3810 m/s (atr.t.) | ||||||||||||||||||||||||||||||||
| Poisson ratio | 0.34 | ||||||||||||||||||||||||||||||||
| Mohs hardness | 3.0 | ||||||||||||||||||||||||||||||||
| Vickers hardness | 343–369 MPa | ||||||||||||||||||||||||||||||||
| Brinell hardness | 235–878 MPa | ||||||||||||||||||||||||||||||||
| CAS Number | 7440-50-8 | ||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||
| Naming | afterCyprus,principal mining place in Roman era (Cyprium) | ||||||||||||||||||||||||||||||||
| Discovery | Middle East(9000 BC) | ||||||||||||||||||||||||||||||||
| Symbol | "Cu": from Latincuprum | ||||||||||||||||||||||||||||||||
| Isotopes of copper | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
Copperis achemical element;it hassymbolCu(fromLatincuprum) andatomic number29. It is a soft, malleable, andductilemetal with very highthermalandelectrical conductivity.A freshly exposed surface of pure copper has apinkish-orange color.Copper is used as a conductor of heat and electricity, as abuilding material,and as a constituent of various metalalloys,such assterling silverused injewelry,cupronickelused to make marine hardware andcoins,andconstantanused instrain gaugesandthermocouplesfor temperature measurement.
Copper is one of the few metals that can occur in nature in a directly usable metallic form (native metals). This led to very early human use in several regions, fromc. 8000 BC.Thousands of years later, it was the first metal to besmeltedfrom sulfide ores,c. 5000 BC;the first metal to be cast into a shape in a mold,c. 4000 BC;and the first metal to be purposely alloyed with another metal,tin,to createbronze,c. 3500 BC.[8]
Commonly encountered compounds are copper(II) salts, which often impart blue or green colors to such minerals asazurite,malachite,andturquoise,and have been used widely and historically as pigments.
Copper used in buildings, usually for roofing, oxidizes to form a greenpatinaof compounds calledverdigris.Copper is sometimes used indecorative art,both in its elemental metal form and in compounds as pigments. Copper compounds are used asbacteriostatic agents,fungicides,andwood preservatives.
Copper is essential to all living organisms as a tracedietary mineralbecause it is a key constituent of the respiratory enzyme complexcytochrome c oxidase.Inmolluscsandcrustaceans,copper is a constituent of the blood pigmenthemocyanin,replaced by the iron-complexedhemoglobinin fish and othervertebrates.In humans, copper is found mainly in the liver, muscle, and bone.[9]The adult body contains between 1.4 and 2.1 mg of copper per kilogram of body weight.[10]
Etymology
In theRoman era,copper was mined principally onCyprus,the origin of the name of the metal, fromaes cyprium(metal of Cyprus), later corrupted tocuprum(Latin).Coper(Old English) andcopperwere derived from this, the later spelling first used around 1530.[11]
Characteristics
Physical


Copper,silver,andgoldare ingroup 11of the periodic table; these three metals have one s-orbital electron on top of a filled d-electron shelland are characterized by highductility,and electrical and thermal conductivity. The filled d-shells in these elements contribute little to interatomic interactions, which are dominated by the s-electrons throughmetallic bonds.Unlike metals with incomplete d-shells, metallic bonds in copper are lacking acovalentcharacter and are relatively weak. This observation explains the lowhardnessand high ductility ofsingle crystalsof copper.[12]At the macroscopic scale, introduction of extended defects to thecrystal lattice,such as grain boundaries, hinders flow of the material under applied stress, thereby increasing its hardness. For this reason, copper is usually supplied in a fine-grainedpolycrystallineform, which has greater strength than monocrystalline forms.[13]
The softness of copper partly explains its high electrical conductivity (59.6×106S/m) and high thermal conductivity, second highest (second only to silver) among pure metals at room temperature.[14]This is because the resistivity to electron transport in metals at room temperature originates primarily from scattering of electrons on thermal vibrations of the lattice, which are relatively weak in a soft metal.[12]The maximum possible current density of copper in open air is approximately3.1×106A/m2,above which it begins to heat excessively.[15]
Copper is one of a few metallic elements with a natural color other than gray or silver.[16]Pure copper is orange-red and acquires a reddishtarnishwhen exposed to air. This is due to the lowplasma frequencyof the metal, which lies in the red part of the visible spectrum, causing it to absorb the higher-frequency green and blue colors.[17]
As with other metals, if copper is put in contact with another metal in the presence of anelectrolyte,galvanic corrosionwill occur.[18]
Chemical


Copper does not react with water, but it does slowly react with atmospheric oxygen to form a layer of brown-black copper oxide which, unlike therustthat forms on iron in moist air, protects the underlying metal from further corrosion (passivation). A green layer ofverdigris(copper carbonate) can often be seen on old copper structures, such as the roofing of many older buildings[19]and theStatue of Liberty.[20]Coppertarnisheswhen exposed to somesulfurcompounds, with which it reacts to form variouscopper sulfides.[21]
Isotopes
There are 29isotopesof copper.63
Cu
and65
Cu
are stable, with63
Cu
comprising approximately 69% of naturally occurring copper; both have aspinof3⁄2.[22]The other isotopes areradioactive,with the most stable being67
Cu
with ahalf-lifeof 61.83 hours.[22]Sevenmetastable isomershave been characterized;68m
Cu
is the longest-lived with a half-life of 3.8 minutes. Isotopes with amass numberabove 64 decay byβ−,whereas those with a mass number below 64 decay byβ+.64
Cu
,which has a half-life of 12.7 hours, decays both ways.[23]
62
Cu
and64
Cu
have significant applications.62
Cu
is used in62
Cu
Cu-PTSM as aradioactive tracerforpositron emission tomography.[24]
Occurrence

Copper is produced in massive stars[25]and is present in the Earth's crust in a proportion of about 50parts per million(ppm).[26]In nature, copper occurs in a variety of minerals, includingnative copper,copper sulfides such aschalcopyrite,bornite,digenite,covellite,andchalcocite,coppersulfosaltssuch astetrahedite-tennantite,andenargite,copper carbonates such asazuriteandmalachite,and as copper(I) or copper(II) oxides such ascupriteandtenorite,respectively.[14]The largest mass of elemental copper discovered weighed 420 tonnes and was found in 1857 on theKeweenaw Peninsulain Michigan, US.[26]Native copper is apolycrystal,with the largest single crystal ever described measuring4.4 × 3.2 × 3.2 cm.[27]Copper is the 25th most abundant element inEarth's crust,representing 50 ppm compared with 75 ppm forzinc,and 14 ppm forlead.[28]
Typical background concentrations of copper do not exceed1 ng/m3in the atmosphere;150 mg/kgin soil;30 mg/kgin vegetation; 2 μg/L in freshwater and0.5 μg/Lin seawater.[29]
Production

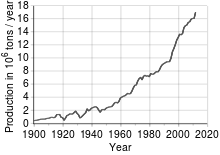
Most copper is mined orextractedas copper sulfides from largeopen pit minesinporphyry copperdeposits that contain 0.4 to 1.0% copper. Sites includeChuquicamata,in Chile,Bingham Canyon Mine,in Utah, United States, andEl Chino Mine,in New Mexico, United States. According to theBritish Geological Survey,in 2005, Chile was the top producer of copper with at least one-third of the world share followed by the United States, Indonesia and Peru.[14]Copper can also be recovered through thein-situ leachprocess. Several sites in the state of Arizona are considered prime candidates for this method.[30]The amount of copper in use is increasing and the quantity available is barely sufficient to allow all countries to reach developed world levels of usage.[31]An alternative source of copper forcollectioncurrently being researched arepolymetallic nodules,which are located at the depths of thePacific Oceanapproximately 3000–6500 meters below sea level. These nodules contain other valuable metals such ascobaltandnickel.[32]
Reserves and prices
Copper has been in use for at least 10,000 years, but more than 95% of all copper ever mined andsmeltedhas been extracted since 1900.[33]As with many natural resources, the total amount of copper on Earth is vast, with around 1014tons in the top kilometer of Earth's crust, which is about 5 million years' worth at the current rate of extraction. However, only a tiny fraction of these reserves is economically viable with present-day prices and technologies. Estimates of copper reserves available for mining vary from 25 to 60 years, depending on core assumptions such as the growth rate.[34]Recycling is a major source of copper in the modern world.[33]

The price of copper isvolatile.[35]After a peak in 2022 the price unexpectedly fell.[36]
Methods

The great majority of copper ores are sulfides. Common ores are the sulfides chalcopyrite (CuFeS2), bornite (Cu5FeS4) and, to a lesser extent, covellite (CuS) and chalcocite (Cu2S).[37]These ores occur at the level of <1% Cu. Concentration of the ore is required, which begins withcomminutionfollowed byfroth flotation.The remaining concentrate is the smelted, which can be described with two simplified equations: [38]
- 2 Cu2S + 3 O2→ 2 Cu2O + 2 SO2
Cuprous oxide reacts with cuprous sulfide to convert toblistercopper upon heating
- 2 Cu2O + Cu2S → 6 Cu + 2 SO2
This roasting gives matte copper, roughly 50% Cu by weight, which is purified by electrolysis. Depending on the ore, sometimes other metals are obtained during the electrolysis including platinum and gold.
Aside from sulfides, another family of ores are oxides. Approximately 15% of the world's copper supply derives from these oxides. The beneficiation process for oxides involves extraction with sulfuric acid solutions followed by electrolysis. In parallel with the above method for "concentrated" sulfide and oxide ores, copper is recovered frommine tailingsand heaps. A variety of methods are used including leaching with sulfuric acid, ammonia, ferric chloride. Biological methods are also used.[38][39]
A significant source of copper is from recycling. Recycling is facilitated because copper is usually deployed in its metallic state. In 2001, a typical automobile contained 20–30 kg of copper. Recycling usually begins with some melting process using a blast furnace.[38]
A potential source of copper is polymetallic nodules, which have an estimated concentration 1.3%.[40][41]
- Blister copper
- Smelting
- Reverberatory furnace
- Slagremoval
- Copper casting ofanodes
- Casting wheel
- Anodes removal machine
- Anodes take-off
- Rail cars
- Transportation to the tank house

Recycling
Likealuminium,copper is recyclable without any loss of quality, both from raw state and from manufactured products.[42]In volume, copper is the third most recycled metal after iron and aluminium.[43]An estimated 80% of all copper ever mined is still in use today.[44]According to theInternational Resource Panel'sMetal Stocks in Society report,the global per capita stock of copper in use in society is 35–55 kg. Much of this is in more-developed countries (140–300 kg per capita) rather than less-developed countries (30–40 kg per capita).
The process of recycling copper is roughly the same as is used to extract copper but requires fewer steps. High-purity scrap copper is melted in afurnaceand thenreducedand cast intobilletsandingots;lower-purity scrap is refined byelectroplatingin a bath ofsulfuric acid.[45]
Environmental impacts
The environmental cost of copper mining was estimated at 3.7 kgCO2eqper kg of copper in 2019.[46]Codelco, a major producer in Chile, reported that in 2020 the company emitted 2.8t CO2eq per ton (2.8 kg CO2eq per kg) of fine copper.[47]Greenhouse gas emissionsprimarily arise from electricity consumed by the company, especially when sourced from fossil fuels, and from engines required for copper extraction and refinement. Companies that mine land often mismanage waste, rendering the area sterile for life. Additionally, nearby rivers and forests are also negatively impacted. ThePhilippinesis an example of a region where land is overexploited by mining companies.[48]
Copper mining waste in Valea Şesei, Romania, has significantly altered nearby water properties. The water in the affected areas is highly acidic, with a pH range of 2.1–4.9, and shows elevated electrical conductivity levels between 280 and 1561 mS/cm.[49]These changes in water chemistry make the environment inhospitable for fish, essentially rendering the water uninhabitable for aquatic life.
Alloys

Numerous copperalloyshave been formulated, many with important uses.Brassis an alloy of copper andzinc.Bronzeusually refers to copper-tinalloys, but can refer to any alloy of copper such asaluminium bronze.Copper is one of the most important constituents of silver andkaratgold solders used in the jewelry industry, modifying the color, hardness and melting point of the resulting alloys.[52]Some lead-freesoldersconsist of tin alloyed with a small proportion of copper and other metals.[53]
The alloy of copper andnickel,calledcupronickel,is used in low-denomination coins, often for the outer cladding. The US five-cent coin (currently called anickel) consists of 75% copper and 25% nickel in homogeneous composition. Prior to the introduction of cupronickel, which was widely adopted by countries in the latter half of the 20th century,[54]alloys of copper andsilverwere also used, with the United States using an alloy of 90% silver and 10% copper until 1965, when circulating silver was removed from all coins with the exception of the half dollar—these were debased to an alloy of 40% silver and 60% copper between 1965 and 1970.[55]The alloy of 90% copper and 10% nickel, remarkable for its resistance to corrosion, is used for various objects exposed to seawater, though it is vulnerable to the sulfides sometimes found in polluted harbors and estuaries.[56]Alloys of copper with aluminium (about 7%) have a golden color and are used in decorations.[26]Shakudōis a Japanese decorative alloy of copper containing a low percentage of gold, typically 4–10%, that can bepatinatedto a dark blue or black color.[57]
Compounds

Copper forms a rich variety of compounds, usually withoxidation states+1 and +2, which are often calledcuprousandcupric,respectively.[58]Copper compounds promote or catalyse numerous chemical and biological processes.[59]
Binary compounds
As with other elements, the simplest compounds of copper are binary compounds, i.e. those containing only two elements, the principal examples being oxides, sulfides, andhalides.Bothcuprousandcupric oxidesare known. Among the numerouscopper sulfides,[60]important examples includecopper(I) sulfide(Cu2S) andcopper monosulfide(CuS).[61]
Cuprous halides withfluorine,chlorine,bromine,andiodineare known, as are cupric halides withfluorine,chlorine,andbromine.Attempts to prepare copper(II) iodide yield only copper(I) iodide and iodine.[58]
- 2 Cu2++ 4 I−→ 2 CuI + I2
Coordination chemistry

Copper formscoordination complexeswithligands.In aqueous solution, copper(II) exists as[Cu(H
2O)
6]2+
.This complex exhibits the fastest water exchange rate (speed of water ligands attaching and detaching) for any transitionmetal aquo complex.Adding aqueoussodium hydroxidecauses the precipitation of light blue solidcopper(II) hydroxide.A simplified equation is:
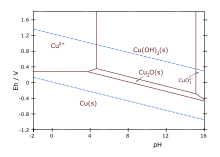
- Cu2++ 2 OH−→ Cu(OH)2
Aqueous ammoniaresults in the same precipitate. Upon adding excess ammonia, the precipitate dissolves, formingtetraamminecopper(II):
- Cu(H
2O)
4(OH)
2+ 4 NH3→[Cu(H
2O)
2(NH
3)
4]2+
+ 2 H2O + 2 OH−
Many otheroxyanionsform complexes; these includecopper(II) acetate,copper(II) nitrate,andcopper(II) carbonate.Copper(II) sulfateforms a blue crystalline pentahydrate,the most familiar copper compound in the laboratory. It is used in afungicidecalled theBordeaux mixture.[62]

Polyols,compounds containing more than one alcoholfunctional group,generally interact with cupric salts. For example, copper salts are used to test forreducing sugars.Specifically, usingBenedict's reagentandFehling's solutionthe presence of the sugar is signaled by a color change from blue Cu(II) to reddish copper(I) oxide.[63]Schweizer's reagent and related complexes withethylenediamineand otheraminesdissolvecellulose.[64]Amino acidssuch as cystine form very stablechelate complexeswith copper(II)[65][66][67]including in the form ofmetal-organic biohybrids(MOBs). Many wet-chemical tests for copper ions exist, one involvingpotassium ferricyanide,which gives a red-brown precipitate with copper(II) salts.[68]
Organocopper chemistry
Compounds that contain a carbon-copper bond are known as organocopper compounds. They are very reactive towards oxygen to form copper(I) oxide and havemany uses in chemistry.They are synthesized by treating copper(I) compounds withGrignard reagents,terminal alkynesororganolithium reagents;[69]in particular, the last reaction described produces aGilman reagent.These can undergosubstitutionwithalkyl halidesto formcoupling products;as such, they are important in the field oforganic synthesis.Copper(I) acetylideis highly shock-sensitive but is an intermediate in reactions such as theCadiot–Chodkiewicz coupling[70]and theSonogashira coupling.[71]Conjugate additiontoenones[72]andcarbocuprationof alkynes[73]can also be achieved with organocopper compounds. Copper(I) forms a variety of weak complexes withalkenesandcarbon monoxide,especially in the presence of amine ligands.[74]
Copper(III) and copper(IV)
Copper(III) is most often found in oxides. A simple example is potassiumcuprate,KCuO2,a blue-black solid.[75]The most extensively studied copper(III) compounds are thecuprate superconductors.Yttrium barium copper oxide(YBa2Cu3O7) consists of both Cu(II) and Cu(III) centres. Like oxide,fluorideis a highlybasicanion[76]and is known to stabilize metal ions in high oxidation states. Both copper(III) and even copper(IV) fluorides are known,K3CuF6andCs2CuF6,respectively.[58]
Some copper proteins formoxo complexes,which, in extensively studied synthetic analog systems, feature copper(III).[77][78]Withtetrapeptides,purple-colored copper(III) complexes are stabilized by the deprotonatedamideligands.[79]
Complexes of copper(III) are also found as intermediates in reactions of organocopper compounds, for example in theKharasch–Sosnovsky reaction.[80][81][82]
History
A timeline of copper illustrates how this metal has advanced human civilization for the past 11,000 years.[83]
Prehistoric
Copper Age



Copper occurs naturally asnative metallic copperand was known to some of the oldest civilizations on record. The history of copper use dates to 9000 BC in the Middle East;[84]a copper pendant was found in northern Iraq that dates to 8700 BC.[85]Evidence suggests that gold andmeteoric iron(but not smelted iron) were the only metals used by humans before copper.[86]The history of copper metallurgy is thought to follow this sequence: first,cold workingof native copper, thenannealing,smelting,and, finally,lost-wax casting.In southeasternAnatolia,all four of these techniques appear more or less simultaneously at the beginning of theNeolithicc. 7500 BC.[87]
Copper smelting was independently invented in different places. The earliest evidence oflost-wax castingcopper comes from an amulet found inMehrgarh,Pakistan, and is dated to 4000 BC.[88]Investment castingwas invented in 4500–4000 BC in Southeast Asia[84]Smelting was probably discovered in China before 2800 BC, in Central America around 600 AD, and in West Africa about the 9th or 10th century AD.[89]Carbon datinghas established mining atAlderley EdgeinCheshire,UK, at 2280 to 1890 BC.[90]
Ötzi the Iceman,a male dated from 3300 to 3200 BC, was found with an axe with a copper head 99.7% pure; high levels ofarsenicin his hair suggest an involvement in copper smelting.[91]Experience with copper has assisted the development of other metals; in particular, copper smelting likely led to the discovery ofiron smelting.[91]

Production in theOld Copper Complexin Michigan and Wisconsin is dated between 6500 and 3000 BC.[92][93][94]A copper spearpoint found in Wisconsin has been dated to 6500 BC.[92]Copper usage by the indigenous peoples of the Old Copper Complex from theGreat Lakes regionof North America has been radiometrically dated to as far back as 7500 BC.[92][95][96]Indigenous peoples of North America around theGreat Lakesmay have also been mining copper during this time, making it one of the oldest known examples ofcopper extractionin the world.[97]There is evidence from prehistoric lead pollution from lakes in Michigan that people in the region began mining copperc. 6000 BC.[97][92]Evidence suggests that utilitarian copper objects fell increasingly out of use in the Old Copper Complex of North America during the Bronze Age and a shift towards an increased production of ornamental copper objects occurred.[98]
Bronze Age

Natural bronze, a type of copper made from ores rich in silicon, arsenic, and (rarely) tin, came into general use in the Balkans around 5500 BC.[99]Alloying copper with tin to make bronze was first practiced about 4000 years after the discovery of copper smelting, and about 2000 years after "natural bronze" had come into general use.[100]Bronze artifacts from theVinča culturedate to 4500 BC.[101]SumerianandEgyptianartifacts of copper and bronze alloys date to 3000 BC.[102]Egyptian Blue,or cuprorivaite (calcium copper silicate) is a synthetic pigment that contains copper and started being used inancient Egyptaround 3250 BC.[103]The manufacturing process of Egyptian blue was known to the Romans, but by the fourth century AD the pigment fell out of use and the secret to its manufacturing process became lost. The Romans said the blue pigment was made from copper, silica, lime andnatronand was known to them ascaeruleum.
TheBronze Agebegan in Southeastern Europe around 3700–3300 BC, in Northwestern Europe about 2500 BC. It ended with the beginning of the Iron Age, 2000–1000 BC in the Near East, and 600 BC in Northern Europe. The transition between theNeolithicperiod and the Bronze Age was formerly termed theChalcolithicperiod (copper-stone), when copper tools were used with stone tools. The term has gradually fallen out of favor because in some parts of the world, the Chalcolithic and Neolithic are coterminous at both ends. Brass, an alloy of copper and zinc, is of much more recent origin. It was known to the Greeks, but became a significant supplement to bronze during the Roman Empire.[102]
Ancient and post-classical


In Greece, copper was known by the namechalkos(χαλκός). It was an important resource for the Romans, Greeks and other ancient peoples. In Roman times, it was known asaes Cyprium,aesbeing the generic Latin term for copper alloys andCypriumfromCyprus,where much copper was mined. The phrase was simplified tocuprum,hence the Englishcopper.Aphrodite(Venusin Rome) represented copper in mythology and alchemy because of its lustrous beauty and its ancient use in producing mirrors; Cyprus, the source of copper, was sacred to the goddess. The seven heavenly bodies known to the ancients were associated with the seven metals known in antiquity, and Venus was assigned to copper, both because of the connection to the goddess and because Venus was the brightest heavenly body after the Sun and Moon and so corresponded to the most lustrous and desirable metal after gold and silver.[104]
Copper was first mined in ancient Britain as early as 2100 BC. Mining at the largest of these mines, theGreat Orme,continued into the late Bronze Age. Mining seems to have been largely restricted tosupergeneores, which were easier to smelt. The rich copper deposits ofCornwallseem to have been largely untouched, in spite of extensivetinmining in the region, for reasons likely social and political rather than technological.[105]
In North America, native copper is known to have been extracted from sites onIsle Royalewith primitive stone tools between 800 and 1600 AD.[106]Copper annealing was being performed in the North American city ofCahokiaaround 1000–1300 AD.[107]There are several exquisite copper plates, known as theMississippian copper platesthat have been found in North America in the area around Cahokia dating from this time period (1000–1300 AD).[107]The copper plates were thought to have been manufactured at Cahokia before ending up elsewhere in the Midwest and southeastern United States like theWulfing cacheandEtowah plates.

In South America a copper mask dated to 1000 BC found in the Argentinian Andes is the oldest known copper artifact discovered in the Andes.[108]Peru has been considered the origin for early coppermetallurgy in pre-Columbian America,but the copper mask from Argentina suggests that theCajón del Maipoof the southern Andes was another important center for early copper workings in South America.[108]Copper metallurgy was flourishing in South America, particularly in Peru around 1000 AD. Copper burial ornamentals from the 15th century have been uncovered, but the metal's commercial production did not start until the early 20th century.[citation needed]
The cultural role of copper has been important, particularly in currency. Romans in the 6th through 3rd centuries BC used copper lumps as money. At first, the copper itself was valued, but gradually the shape and look of the copper became more important.Julius Caesarhad his own coins made from brass, whileOctavianus Augustus Caesar's coins were made from Cu-Pb-Sn alloys. With an estimated annual output of around 15,000 t,Roman copper mining and smelting activitiesreached a scale unsurpassed until the time of theIndustrial Revolution;theprovincesmost intensely mined were those ofHispania,Cyprus and in Central Europe.[109][110]
The gates of theTemple of JerusalemusedCorinthian bronzetreated withdepletion gilding.[clarification needed][citation needed]The process was most prevalent inAlexandria,where alchemy is thought to have begun.[111]In ancientIndia,copper was used in theholisticmedical scienceAyurvedaforsurgicalinstruments and other medical equipment.Ancient Egyptians(~2400 BC) used copper for sterilizing wounds and drinking water, and later to treat headaches, burns, and itching.[citation needed]
Modern


TheGreat Copper Mountainwas a mine in Falun, Sweden, that operated from the 10th century to 1992. It satisfied two-thirds of Europe's copper consumption in the 17th century and helped fund many of Sweden's wars during that time.[112]It was referred to as the nation's treasury; Sweden had acopper backed currency.[113]

Copper is used in roofing,[19]currency, and for photographic technology known as thedaguerreotype.Copper was used inRenaissancesculpture, and was used to construct theStatue of Liberty;copper continues to be used in construction of various types.Copper platingandcopper sheathingwere widely used to protect the under-water hulls of ships, a technique pioneered by the British Admiralty in the 18th century.[114]TheNorddeutsche Affineriein Hamburg was the first modernelectroplatingplant, starting its production in 1876.[115]The German scientistGottfried Osanninventedpowder metallurgyin 1830 while determining the metal's atomic mass; around then it was discovered that the amount and type of alloying element (e.g., tin) to copper would affect bell tones.[citation needed]
During the rise in demand for copper for the Age of Electricity, from the 1880s until the Great Depression of the 1930s, the United States produced one third to half the world's newly mined copper.[116]Major districts included the Keweenaw district of northern Michigan, primarily native copper deposits, which was eclipsed by the vast sulphide deposits ofButte, Montana,in the late 1880s, which itself was eclipsed by porphyry deposits of the Southwest United States, especially atBingham Canyon, Utah,andMorenci, Arizona.Introduction of open pit steam shovel mining and innovations in smelting, refining, flotation concentration and other processing steps led to mass production. Early in the twentieth century,Arizonaranked first, followed byMontana,thenUtahandMichigan.[117]
Flash smeltingwas developed byOutokumpuin Finland and first applied atHarjavaltain 1949; the energy-efficient process accounts for 50% of the world's primary copper production.[118]
TheIntergovernmental Council of Copper Exporting Countries,formed in 1967 by Chile, Peru, Zaire and Zambia, operated in the copper market asOPECdoes in oil, though it never achieved the same influence, particularly because the second-largest producer, the United States, was never a member; it was dissolved in 1988.[119]
Applications

The major applications of copper are electrical wire (60%), roofing and plumbing (20%), and industrial machinery (15%). Copper is used mostly as a pure metal, but when greater hardness is required, it is put into such alloys asbrassandbronze(5% of total use).[26]For more than two centuries, copper paint has been used on boat hulls to control the growth of plants and shellfish.[120]A small part of the copper supply is used for nutritional supplements and fungicides in agriculture.[62][121]Machiningof copper is possible, although alloys are preferred for goodmachinabilityin creating intricate parts.
Wire and cable
Despite competition from other materials, copper remains the preferredelectrical conductorin nearly all categories of electrical wiring except overheadelectric power transmissionwherealuminiumis often preferred.[122][123]Copper wire is used inpower generation,power transmission,power distribution,telecommunications,electronicscircuitry, and countless types ofelectrical equipment.[124]Electrical wiringis the most important market for the copper industry.[125]This includes structural power wiring, power distribution cable, appliance wire, communications cable, automotive wire and cable, and magnet wire. Roughly half of all copper mined is used for electrical wire and cable conductors.[126]Many electrical devices rely on copper wiring because of its multitude of inherent beneficial properties, such as its highelectrical conductivity,tensile strength,ductility,creep (deformation)resistance,corrosionresistance, lowthermal expansion,highthermal conductivity,ease ofsoldering,malleability,and ease of installation.
For a short period from the late 1960s to the late 1970s, copper wiring was replaced byaluminium wiringin many housing construction projects in America. The new wiring was implicated in a number of house fires and the industry returned to copper.[127]

Integrated circuitsandprinted circuit boardsincreasingly feature copper in place of aluminium because of its superior electrical conductivity;heat sinksandheat exchangersuse copper because of its superior heat dissipation properties.Electromagnets,vacuum tubes,cathode ray tubes,andmagnetronsin microwave ovens use copper, as dowaveguidesfor microwave radiation.[128]
Electric motors
Copper's superiorconductivityenhances the efficiency of electricalmotors.[129]This is important because motors and motor-driven systems account for 43–46% of all global electricity consumption and 69% of all electricity used by industry.[130]Increasing the mass and cross section of copper in acoilincreases the efficiency of the motor.Copper motor rotors,a new technology designed for motor applications where energy savings are prime design objectives,[131][132]are enabling general-purposeinduction motorsto meet and exceedNational Electrical Manufacturers Association(NEMA)premium efficiencystandards.[133]
Renewable energy production
Renewable energysources such assolar,wind,tidal,hydro,biomass,andgeothermalhave become significant sectors of the energy market.[134][135]The rapid growth of these sources in the 21st century has been prompted by increasing costs offossil fuelsas well as theirenvironmental impactissues thatsignificantly loweredtheir use.
Copper plays an important role in these renewable energy systems.[136][137][138][139][140]Copper usage averages up to five times more in renewable energy systems than in traditional power generation, such as fossil fuel andnuclearpower plants.[141]Since copper is an excellentthermalandelectrical conductoramong engineering metals (second only to silver),[142]electrical systems that utilize copper generate and transmit energy with high efficiency and with minimum environmental impacts.
When choosing electrical conductors, facility planners and engineers factor capital investment costs of materials against operational savings due to their electrical energy efficiencies over their useful lives, plus maintenance costs. Copper often fares well in these calculations. A factor called "copper usage intensity,” is a measure of the quantity of copper necessary to install one megawatt of new power-generating capacity.

When planning for a new renewable power facility, engineers and product specifiers seek to avoid supply shortages of selected materials. According to theUnited States Geological Survey,in-ground copperreserveshave increased more than 700% since 1950, from almost 100 million tonnes to 720 million tonnes in 2017, despite the fact that world refined usage has more than tripled in the last 50 years.[143]Copper resources are estimated to exceed 5,000 million tonnes.[144][145]
Bolstering the supply fromcopper extractionis the more than 30 percent of copper installed from 2007 to 2017 that came from recycled sources.[146]Itsrecycling rateis higher than any other metal.[147]Architecture



Copper has been used since ancient times as a durable,corrosion resistant,and weatherproof architectural material.[148][149][150][151]Roofs,flashings,rain gutters,downspouts,domes,spires,vaults, anddoorshave been made from copper for hundreds or thousands of years. Copper's architectural use has been expanded in modern times to include interior and exteriorwall cladding,buildingexpansion joints,radio frequency shielding,andantimicrobialand decorative indoor products such as attractive handrails, bathroom fixtures, and counter tops. Some of copper's other important benefits as an architectural material include lowthermal movement,light weight,lightning protection,and recyclability.
The metal's distinctive natural greenpatinahas long been coveted by architects and designers. The final patina is a particularly durable layer that is highly resistant to atmospheric corrosion, thereby protecting the underlying metal against further weathering.[152][153][154]It can be a mixture of carbonate and sulfate compounds in various amounts, depending upon environmental conditions such as sulfur-containing acid rain.[155][156][157][158]Architectural copper and its alloys can also be'finished'to take on a particular look, feel, or color. Finishes include mechanical surface treatments, chemical coloring, and coatings.[159]
Copper has excellentbrazingandsolderingproperties and can bewelded;the best results are obtained withgas metal arc welding.[160]
Antibiofouling
Copper isbiostatic,meaning bacteria and many other forms of life will not grow on it. For this reason it has long been used to line parts of ships to protect againstbarnaclesandmussels.It was originally used pure, but has since been superseded byMuntz metaland copper-based paint. Similarly, as discussed incopper alloys in aquaculture,copper alloys have become important netting materials in theaquacultureindustry because they areantimicrobialand preventbiofouling,even in extreme conditions[161]and have strong structural andcorrosion-resistant[162]properties in marine environments.
Antimicrobial
Copper-alloy touch surfaceshave natural properties that destroy a wide range ofmicroorganisms(e.g.,E. coliO157:H7,methicillin-resistantStaphylococcus aureus(MRSA),Staphylococcus,Clostridium difficile,influenza A virus,adenovirus,SARS-CoV-2,andfungi).[163][164]Indians have been using copper vessels since ancient times for storing water, even before modern science realized its antimicrobial properties.[165]Some copper alloys were proven to kill more than 99.9% of disease-causing bacteria within just two hours when cleaned regularly.[166]TheUnited States Environmental Protection Agency(EPA) has approved the registrations of these copper alloys as "antimicrobialmaterials with public health benefits ";[166]that approval allows manufacturers to make legal claims to the public health benefits of products made of registered alloys. In addition, the EPA has approved a long list of antimicrobial copper products made from these alloys, such as bedrails,handrails,over-bed tables,sinks,faucets,door knobs,toilethardware,computer keyboards,health clubequipment, andshopping carthandles. Copper doorknobs are used by hospitals to reduce the transfer of disease, andLegionnaires' diseaseis suppressed by copper tubing in plumbing systems.[167]Antimicrobial copper alloy products are now being installed in healthcare facilities in the U.K., Ireland, Japan, Korea, France, Denmark, and Brazil, as well as being called for in the US,[168]and in the subway transit system in Santiago, Chile, where copper–zinc alloy handrails were installed in some 30 stations between 2011 and 2014.[169][170][171] Textile fibers can be blended with copper to create antimicrobial protective fabrics.[172][unreliable source?]
Copper demand
Total world production in 2023 is expected to be almost 23 millionmetric tons.[173]Copper demand is increasing due to the ongoingenergy transition to electricity.[174]China accounts for over half the demand.[175]
For some purposes, other metals can substitute,aluminium wirewas substituted in many applications, but improper design resulted in fire hazards.[176]The safety issues have since been solved by use of larger sizes of aluminium wire (#8AWG and up), and properly designed aluminium wiring is still being installed in place of copper. For example, theAirbus A380uses aluminum wire in place of copper wire for electrical power transmission.[177]
Speculative investing
Copper may be used as a speculative investment due to the predicted increase in use from worldwide infrastructure growth, and the important role it has in producingwind turbines,solar panels,and otherrenewable energysources.[178][179]Another reason predicted demand increases is the fact thatelectric carscontain an average of 3.6 times as much copper as conventional cars, although the effect ofelectric carson copper demand is debated.[180][181]Some people invest in copper through copper mining stocks,ETFs,andfutures.Others store physical copper in the form of copper bars or rounds although these tend to carry a higher premium in comparison to precious metals.[182]Those who want to avoid the premiums of copperbullionalternatively store oldcopper wire,copper tubingor Americanpennies made before 1982.[183]
Folk medicine
Copper is commonly used in jewelry, and according to some folklore, copper bracelets relievearthritissymptoms.[184]In one trial for osteoarthritis and one trial for rheumatoid arthritis, no differences were found between copper bracelet and control (non-copper) bracelet.[185][186]No evidence shows that copper can be absorbed through the skin. If it were, it might lead tocopper poisoning.[187]
Degradation
Chromobacterium violaceumandPseudomonas fluorescenscan both mobilize solid copper as a cyanide compound.[188]The ericoid mycorrhizal fungi associated withCalluna,EricaandVacciniumcan grow in metalliferous soils containing copper.[188]The ectomycorrhizal fungusSuillus luteusprotects young pine trees from copper toxicity. A sample of the fungusAspergillus nigerwas found growing from gold mining solution and was found to contain cyano complexes of such metals as gold, silver, copper, iron, and zinc. The fungus also plays a role in the solubilization of heavy metal sulfides.[189]
Biological role

Biochemistry
Copper proteinshave diverse roles in biological electron transport and oxygen transportation, processes that exploit the easy interconversion of Cu(I) and Cu(II).[190]Copper is essential in the aerobicrespirationof alleukaryotes.Inmitochondria,it is found incytochrome c oxidase,which is the last protein inoxidative phosphorylation.Cytochrome c oxidase is the protein that binds the O2between a copper and an iron; the protein transfers 8 electrons to the O2molecule to reduce it to two molecules of water. Copper is also found in manysuperoxide dismutases,proteins that catalyze the decomposition ofsuperoxidesby converting it (bydisproportionation) to oxygen andhydrogen peroxide:
- Cu2+-SOD + O2−→ Cu+-SOD + O2(reduction of copper; oxidation of superoxide)
- Cu+-SOD + O2−+ 2H+→ Cu2+-SOD + H2O2(oxidation of copper; reduction of superoxide)
The proteinhemocyaninis the oxygen carrier in mostmollusksand somearthropodssuch as thehorseshoe crab(Limulus polyphemus).[191]Because hemocyanin is blue, these organisms have blue blood rather than the red blood of iron-basedhemoglobin.Structurally related to hemocyanin are thelaccasesandtyrosinases.Instead of reversibly binding oxygen, these proteins hydroxylate substrates, illustrated by their role in the formation oflacquers.[192]The biological role for copper commenced with the appearance of oxygen in Earth's atmosphere.[193]Several copper proteins, such as the "blue copper proteins", do not interact directly with substrates; hence they are not enzymes. These proteins relay electrons by the process calledelectron transfer.[192]
A unique tetranuclear copper center has been found innitrous-oxide reductase.[194]
Chemical compounds which were developed for treatment of Wilson's disease have been investigated for use in cancer therapy.[195]
Nutrition
Copper is an essentialtrace elementin plants and animals, but not all microorganisms. The human body contains copper at a level of about 1.4 to 2.1 mg per kg of body mass.[196]
Absorption
Copper is absorbed in the gut, then transported to the liver bound toalbumin.[197]After processing in the liver, copper is distributed to other tissues in a second phase, which involves the proteinceruloplasmin,carrying the majority of copper in blood. Ceruloplasmin also carries the copper that is excreted in milk, and is particularly well-absorbed as a copper source.[198]Copper in the body normally undergoesenterohepatic circulation(about 5 mg a day, vs. about 1 mg per day absorbed in the diet and excreted from the body), and the body is able to excrete some excess copper, if needed, viabile,which carries some copper out of the liver that is not then reabsorbed by the intestine.[199][200]
Dietary recommendations
TheU.S. Institute of Medicine(IOM) updated the estimated average requirements (EARs) and recommended dietary allowances (RDAs) for copper in 2001. If there is not sufficient information to establish EARs and RDAs, an estimate designatedAdequate Intake(AI) is used instead. The AIs for copper are: 200 μg of copper for 0–6-month-old males and females, and 220 μg of copper for 7–12-month-old males and females. For both sexes, the RDAs for copper are: 340 μg of copper for 1–3 years old, 440 μg of copper for 4–8 years old, 700 μg of copper for 9–13 years old, 890 μg of copper for 14–18 years old and 900 μg of copper for ages 19 years and older. For pregnancy, 1,000 μg. For lactation, 1,300 μg.[201]As for safety, the IOM also setstolerable upper intake levels(ULs) for vitamins and minerals when evidence is sufficient. In the case of copper the UL is set at 10 mg/day. Collectively the EARs, RDAs, AIs and ULs are referred to asDietary Reference Intakes.[202]
TheEuropean Food Safety Authority(EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL defined the same as in United States. For women and men ages 18 and older the AIs are set at 1.3 and 1.6 mg/day, respectively. AIs for pregnancy and lactation is 1.5 mg/day. For children ages 1–17 years the AIs increase with age from 0.7 to 1.3 mg/day. These AIs are higher than the U.S. RDAs.[203]The European Food Safety Authority reviewed the same safety question and set its UL at 5 mg/day, which is half the U.S. value.[204]
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For copper labeling purposes 100% of the Daily Value was 2.0 mg, but as of May 27, 2016[update],it was revised to 0.9 mg to bring it into agreement with the RDA.[205][206]A table of the old and new adult daily values is provided atReference Daily Intake.
Deficiency
Because of its role in facilitating iron uptake,copper deficiencycan produceanemia-like symptoms,neutropenia,bone abnormalities, hypopigmentation, impaired growth, increased incidence of infections, osteoporosis, hyperthyroidism, and abnormalities in glucose and cholesterol metabolism. Conversely,Wilson's diseasecauses an accumulation of copper in body tissues.
Severe deficiency can be found by testing for low plasma or serum copper levels, low ceruloplasmin, and low red blood cell superoxide dismutase levels; these are not sensitive to marginal copper status. The "cytochrome c oxidase activity of leucocytes and platelets" has been stated as another factor in deficiency, but the results have not been confirmed by replication.[207]
Toxicity
Gram quantities of various copper salts have been taken in suicide attempts and produced acute copper toxicity in humans, possibly due to redox cycling and the generation ofreactive oxygen speciesthat damageDNA.[208][209]Corresponding amounts of copper salts (30 mg/kg) are toxic in animals.[210]A minimum dietary value for healthy growth in rabbits has been reported to be at least 3ppmin the diet.[211]However, higher concentrations of copper (100 ppm, 200 ppm, or 500 ppm) in the diet of rabbits may favorably influencefeed conversion efficiency,growth rates, and carcass dressing percentages.[212]
Chronic copper toxicity does not normally occur in humans because of transport systems that regulate absorption and excretion. Autosomal recessive mutations in copper transport proteins can disable these systems, leading toWilson's diseasewith copper accumulation andcirrhosisof the liver in persons who have inherited two defective genes.[196]
Elevated copper levels have also been linked to worsening symptoms ofAlzheimer's disease.[213][214]
Human exposure
In the US, theOccupational Safety and Health Administration(OSHA) has designated apermissible exposure limit(PEL) for copper dust and fumes in the workplace as a time-weighted average (TWA) of 1 mg/m3.[215]TheNational Institute for Occupational Safety and Health(NIOSH) has set arecommended exposure limit(REL) of 1 mg/m3,time-weighted average. TheIDLH(immediately dangerous to life and health) value is 100 mg/m3.[216]
Copper is a constituent oftobacco smoke.[217][218]Thetobacco plantreadily absorbs and accumulatesheavy metals,such as copper from the surrounding soil into its leaves. These are readily absorbed into the user's body following smoke inhalation.[219]The health implications are not clear.[220]
See also
- Copper in renewable energy
- Copper nanoparticle
- Erosion corrosion of copper water tubes
- List of countries by copper production
- Metal theft
- Anaconda Copper
- Antofagasta PLC
- Codelco
- El Boleo mine
- Grasberg mine
- Copper foil
References
- ^"Standard Atomic Weights: Copper".CIAAW.1969.
- ^Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (4 May 2022)."Standard atomic weights of the elements 2021 (IUPAC Technical Report)".Pure and Applied Chemistry.doi:10.1515/pac-2019-0603.ISSN1365-3075.
- ^abcArblaster, John W. (2018).Selected Values of the Crystallographic Properties of Elements.Materials Park, Ohio: ASM International.ISBN978-1-62708-155-9.
- ^Moret, Marc-Etienne; Zhang, Limei; Peters, Jonas C. (2013)."A Polar Copper–Boron One-Electron σ-Bond".J. Am. Chem. Soc.135(10): 3792–3795.doi:10.1021/ja4006578.PMID23418750.
- ^Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds".CRC Handbook of Chemistry and Physics(PDF)(86th ed.). Boca Raton (FL): CRC Press.ISBN0-8493-0486-5.Archived fromthe original(PDF)on 3 March 2011.
- ^Weast, Robert (1984).CRC, Handbook of Chemistry and Physics.Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110.ISBN0-8493-0464-4.
- ^Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021)."The NUBASE2020 evaluation of nuclear properties"(PDF).Chinese Physics C.45(3): 030001.doi:10.1088/1674-1137/abddae.
- ^Robert McHenry, ed. (1992)."Bronze".The New Encyclopædia Britannica.Vol. 3 (15 ed.). Chicago: Encyclopædia Britannica, Incorporated. p. 612.ISBN978-0-85229-553-3.OCLC25228234.
- ^Johnson, MD PhD, Larry E., ed. (2008)."Copper".Merck Manual Home Health Handbook.Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. Archived fromthe originalon 7 March 2016.Retrieved7 April2013.
- ^"Copper in human health".
- ^"Copper".Merriam-Webster Dictionary. 2018.Retrieved22 August2018.
- ^abTrigg, George L.; Immergut, Edmund H. (1992).Encyclopedia of Applied Physics.Vol. 4: Combustion to Diamagnetism. VCH. pp. 267–272.ISBN978-3-527-28126-8.Retrieved2 May2011.
- ^Smith, William F. & Hashemi, Javad (2003).Foundations of Materials Science and Engineering.McGraw-Hill Professional. p. 223.ISBN978-0-07-292194-6.
- ^abcHammond, C. R. (2004).The Elements, in Handbook of Chemistry and Physics(81st ed.). CRC Press.ISBN978-0-8493-0485-9.
- ^Resistance Welding Manufacturing Alliance (2003).Resistance Welding Manual(4th ed.). Resistance Welding Manufacturing Alliance. pp. 18–12.ISBN978-0-9624382-0-2.
- ^Chambers, William; Chambers, Robert (1884).Chambers's Information for the People.Vol. L (5th ed.). W. & R. Chambers. p. 312.ISBN978-0-665-46912-1.
- ^Ramachandran, Harishankar (14 March 2007)."Why is Copper Red?"(PDF).IIT Madras.Retrieved27 December2022.
- ^"Galvanic Corrosion".Corrosion Doctors.Retrieved29 April2011.
- ^abGrieken, Rene van; Janssens, Koen (2005).Cultural Heritage Conservation and Environmental Impact Assessment by Non-Destructive Testing and Micro-Analysis.CRC Press. p. 197.ISBN978-0-203-97078-2.
- ^"Copper.org: Education: Statue of Liberty: Reclothing the First Lady of Metals – Repair Concerns".Copper.org.Retrieved11 April2011.
- ^Rickett, B. I.; Payer, J. H. (1995). "Composition of Copper Tarnish Products Formed in Moist Air with Trace Levels of Pollutant Gas: Hydrogen Sulfide and Sulfur Dioxide/Hydrogen Sulfide".Journal of the Electrochemical Society.142(11): 3723–3728.Bibcode:1995JElS..142.3723R.doi:10.1149/1.2048404.
- ^abAudi, Georges; Bersillon, Olivier; Blachot, Jean;Wapstra, Aaldert Hendrik(2003),"The NUBASEevaluation of nuclear and decay properties ",Nuclear Physics A,729:3–128,Bibcode:2003NuPhA.729....3A,doi:10.1016/j.nuclphysa.2003.11.001
- ^"Interactive Chart of Nuclides".National Nuclear Data Center.Archived fromthe originalon 25 August 2013.Retrieved8 April2011.
- ^Okazawad, Hidehiko; Yonekura, Yoshiharu; Fujibayashi, Yasuhisa; Nishizawa, Sadahiko; Magata, Yasuhiro; Ishizu, Koichi; Tanaka, Fumiko; Tsuchida, Tatsuro; Tamaki, Nagara; Konishi, Junji (1994)."Clinical Application and Quantitative Evaluation of Generator-Produced Copper-62-PTSM as a Brain Perfusion Tracer for PET"(PDF).Journal of Nuclear Medicine.35(12): 1910–1915.PMID7989968.
- ^Romano, Donatella; Matteucci, Fransesca (2007). "Contrasting copper evolution in ω Centauri and the Milky Way".Monthly Notices of the Royal Astronomical Society: Letters.378(1): L59–L63.arXiv:astro-ph/0703760.Bibcode:2007MNRAS.378L..59R.doi:10.1111/j.1745-3933.2007.00320.x.S2CID14595800.
- ^abcdEmsley, John (2003).Nature's building blocks: an A–Z guide to the elements.Oxford University Press. pp.121–125.ISBN978-0-19-850340-8.Retrieved2 May2011.
- ^Rickwood, P. C. (1981)."The largest crystals"(PDF).American Mineralogist.66:885.
- ^Emsley, John (2003).Nature's building blocks: an A–Z guide to the elements.Oxford University Press. pp. 124, 231, 449, 503.ISBN978-0-19-850340-8.Retrieved2 May2011.
- ^Rieuwerts, John (2015).The Elements of Environmental Pollution.London and New York: Earthscan Routledge. p. 207.ISBN978-0-415-85919-6.OCLC886492996.
- ^Randazzo, Ryan (19 June 2011)."A new method to harvest copper".Azcentral.com. Archived fromthe originalon 21 February 2023.Retrieved25 April2014.
- ^Gordon, R.B.; Bertram, M.; Graedel, T.E. (2006)."Metal stocks and sustainability".Proceedings of the National Academy of Sciences.103(5): 1209–1214.Bibcode:2006PNAS..103.1209G.doi:10.1073/pnas.0509498103.PMC1360560.PMID16432205.
- ^Beaudoin, Yannick C.; Baker, Elaine (December 2013).Deep Sea Minerals: Manganese Nodules, a physical, biological, environmental and technical review.Secretariat of the Pacific Community. pp. 7–18.ISBN978-82-7701-119-6.Retrieved8 February2021.
- ^abLeonard, Andrew (3 March 2006)."Peak copper?".Salon.Retrieved8 March2022.
- ^Brown, Lester (2006).Plan B 2.0: Rescuing a Planet Under Stress and a Civilization in Trouble.New York: W.W. Norton. p.109.ISBN978-0-393-32831-8.
- ^Schmitz, Christopher (1986). "The Rise of Big Business in the World, Copper Industry 1870–1930".Economic History Review.2.39(3): 392–410.doi:10.1111/j.1468-0289.1986.tb00411.x.JSTOR2596347.
- ^"Copper is unexpectedly getting cheaper".The Economist.ISSN0013-0613.Retrieved19 December2023.
- ^Greenwood, Norman N.;Earnshaw, Alan (1997).Chemistry of the Elements(2nd ed.).Butterworth-Heinemann.pp. 1174–1175.ISBN978-0-08-037941-8.
- ^abcLossin, Adalbert (2001). "Copper".Ullmann's Encyclopedia of Industrial Chemistry.doi:10.1002/14356007.a07_471.ISBN9783527303854.
- ^Watling, H.R. (2006)."The bioleaching of sulphide minerals with emphasis on copper sulphides – A review"(PDF).Hydrometallurgy.84(1): 81–108.Bibcode:2006HydMe..84...81W.doi:10.1016/j.hydromet.2006.05.001.Archived fromthe original(PDF)on 18 August 2011.
- ^Su, Kun; Ma, Xiaodong; Parianos, John; Zhao, Baojun (2020)."Thermodynamic and Experimental Study on Efficient Extraction of Valuable Metals from Polymetallic Nodules".Minerals.10(4): 360.Bibcode:2020Mine...10..360S.doi:10.3390/min10040360.
- ^International Seabed Authority."Polymetallic Nodules"(PDF).International Seabed Authority. Archived fromthe original(PDF)on 23 October 2021.Retrieved8 February2021.
- ^Bahadir, Ali Mufit; Duca, Gheorghe (2009).The Role of Ecological Chemistry in Pollution Research and Sustainable Development.Springer.ISBN978-90-481-2903-4.
- ^Green, Dan (2016).The Periodic Table in Minutes.Quercus.ISBN978-1-68144-329-4.
- ^"International Copper Association".Archived fromthe originalon 5 March 2012.Retrieved22 July2009.
- ^"Overview of Recycled Copper"Copper.org.(25 August 2010). Retrieved on 8 November 2011.
- ^"Les opportunités du recyclage du cuivre de haute pureté"[Opportunities for recycling high purity copper](PDF)(in French). WeeeCycling. 17 May 2021.
- ^"Annual Memory 2020"(PDF).Codelco. p. 109.
- ^"Philippines: attention, terrain miné"[Philippines: attention, mined land] (in French). Amnesty International. 9 November 2016.
- ^Rzymski, Piotr; Klimaszyk, Piotr; Marszelewski, Włodzimierz; Borowiak, Dariusz; Mleczek, Mirosław; Nowiński, Kamil; Pius, Bożena; Niedzielski, Przemysław; Poniedziałek, Barbara (25 July 2017)."The chemistry and toxicity of discharge waters from copper mine tailing impoundment in the valley of the Apuseni Mountains in Romania".Environmental Science and Pollution Research International.24(26): 21445–21458.Bibcode:2017ESPR...2421445R.doi:10.1007/s11356-017-9782-y.PMC5579155.PMID28744684.
- ^"Dime".US Mint.Retrieved9 July2019.[permanent dead link]
- ^"Pride and skill – the 10-cent coin".Royal Canadian Mint.Retrieved9 July2019.
- ^"Gold Jewellery Alloys".World Gold Council. Archived fromthe originalon 14 April 2009.Retrieved6 June2009.
- ^Balver Zinn Solder Sn97Cu3Archived7 July 2011 at theWayback Machine.(PDF). balverzinn.com. Retrieved on 8 November 2011.
- ^Deane, D. V."Modern Coinage Systems"(PDF).British Numismatic Society.Retrieved1 July2019.
- ^"What is 90% Silver?".American Precious Metals Exchange(APMEX).Archived fromthe originalon 28 July 2020.Retrieved1 July2019.
- ^Corrosion Tests and Standards.ASTM International. 2005. p. 368.
- ^Oguchi, Hachiro (1983)."Japanese Shakudō: its history, properties and production from gold-containing alloys".Gold Bulletin.16(4): 125–132.doi:10.1007/BF03214636.
- ^abcHolleman, A.F.; Wiberg, N. (2001).Inorganic Chemistry.San Diego: Academic Press.ISBN978-0-12-352651-9.
- ^Trammell, Rachel; Rajabimoghadam, Khashayar; Garcia-Bosch, Isaac (30 January 2019)."Copper-Promoted Functionalization of Organic Molecules: from Biologically Relevant Cu/O2 Model Systems to Organometallic Transformations".Chemical Reviews.119(4): 2954–3031.doi:10.1021/acs.chemrev.8b00368.PMC6571019.PMID30698952.
- ^Wells, A. F. (1984).Structural Inorganic Chemistry(5th ed.). Oxford University Press. pp. 1142–1145.ISBN978-0-19-965763-6.
- ^Greenwood, Norman N.;Earnshaw, Alan (1997).Chemistry of the Elements(2nd ed.).Butterworth-Heinemann.p. 1181.ISBN978-0-08-037941-8.
- ^abWiley-Vch (2 April 2007)."Nonsystematic (Contact) Fungicides".Ullmann's Agrochemicals.Wiley. p. 623.ISBN978-3-527-31604-5.
- ^Ralph L. Shriner, Christine K.F. Hermann, Terence C. Morrill, David Y. Curtin, Reynold C. Fuson "The Systematic Identification of Organic Compounds" 8th edition, J. Wiley, Hoboken.ISBN0-471-21503-1
- ^Saalwächter, Kay; Burchard, Walther; Klüfers, Peter; Kettenbach, G.; Mayer, Peter; Klemm, Dieter; Dugarmaa, Saran (2000). "Cellulose Solutions in Water Containing Metal Complexes".Macromolecules.33(11): 4094–4107.Bibcode:2000MaMol..33.4094S.CiteSeerX10.1.1.951.5219.doi:10.1021/ma991893m.
- ^Deodhar, S., Huckaby, J., Delahoussaye, M. and DeCoster, M.A., 2014, August. High-aspect ratio bio-metallic nanocomposites for cellular interactions. In IOP Conference Series: Materials Science and Engineering (Vol. 64, No. 1, p. 012014).https://iopscience.iop.org/article/10.1088/1757-899X/64/1/012014/meta.
- ^Kelly, K.C., Wasserman, J.R., Deodhar, S., Huckaby, J. and DeCoster, M.A., 2015. Generation of scalable, metallic high-aspect ratio nanocomposites in a biological liquid medium. Journal of Visualized Experiments, (101), p.e52901.https://www.jove.com/t/52901/generation-scalable-metallic-high-aspect-ratio-nanocomposites.
- ^Karan, A., Darder, M., Kansakar, U., Norcross, Z. and DeCoster, M.A., 2018. Integration of a Copper-Containing Biohybrid (CuHARS) with Cellulose for Subsequent Degradation and Biomedical Control. International journal of environmental research and public health, 15(5), p.844.https://www.mdpi.com/1660-4601/15/5/844
- ^"Characteristic Reactions of Copper Ions (Cu²⁺)".Chemistry LibreTexts.3 April 2018.Retrieved27 May2024.
- ^"Modern Organocopper Chemistry" Norbert Krause, Ed., Wiley-VCH, Weinheim, 2002.ISBN978-3-527-29773-3.
- ^Berná, José; Goldup, Stephen; Lee, Ai-Lan; Leigh, David; Symes, Mark; Teobaldi, Gilberto; Zerbetto, Fransesco (26 May 2008). "Cadiot–Chodkiewicz Active Template Synthesis of Rotaxanes and Switchable Molecular Shuttles with Weak Intercomponent Interactions".Angewandte Chemie.120(23): 4464–4468.Bibcode:2008AngCh.120.4464B.doi:10.1002/ange.200800891.
- ^Rafael Chinchilla & Carmen Nájera (2007). "The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry".Chemical Reviews.107(3): 874–922.doi:10.1021/cr050992x.PMID17305399.
- ^"An Addition of an Ethylcopper Complex to 1-Octyne: (E)-5-Ethyl-1,4-Undecadiene"(PDF).Organic Syntheses.64:1. 1986.doi:10.15227/orgsyn.064.0001.Archived fromthe original(PDF)on 19 June 2012.
- ^Kharasch, M.S.; Tawney, P.O. (1941). "Factors Determining the Course and Mechanisms of Grignard Reactions. II. The Effect of Metallic Compounds on the Reaction between Isophorone and Methylmagnesium Bromide".Journal of the American Chemical Society.63(9): 2308–2316.doi:10.1021/ja01854a005.
- ^Imai, Sadako; Fujisawa, Kiyoshi; Kobayashi, Takako; Shirasawa, Nobuhiko; Fujii, Hiroshi; Yoshimura, Tetsuhiko; Kitajima, Nobumasa; Moro-oka, Yoshihiko (1998). "63Cu NMR Study of Copper(I) Carbonyl Complexes with Various Hydrotris(pyrazolyl)borates: Correlation between 63Cu Chemical Shifts and CO Stretching Vibrations ".Inorganic Chemistry.37(12): 3066–3070.doi:10.1021/ic970138r.
- ^G. Brauer, ed. (1963). "Potassium Cuprate (III)".Handbook of Preparative Inorganic Chemistry.Vol. 1 (2nd ed.). NY: Academic Press. p. 1015.
- ^Schwesinger, Reinhard; Link, Reinhard; Wenzl, Peter; Kossek, Sebastian (2006). "Anhydrous phosphazenium fluorides as sources for extremely reactive fluoride ions in solution".Chemistry: A European Journal.12(2): 438–45.doi:10.1002/chem.200500838.PMID16196062.
- ^Mirica, Liviu M.; Ottenwaelder, Xavier; Stack, T. Daniel P. (1 February 2004)."Structure and Spectroscopy of Copper−Dioxygen Complexes".Chemical Reviews.104(2): 1013–1046.doi:10.1021/cr020632z.ISSN0009-2665.PMID14871148.
- ^Lewis, E.A.; Tolman, W.B. (2004). "Reactivity of Dioxygen-Copper Systems".Chemical Reviews.104(2): 1047–1076.doi:10.1021/cr020633r.PMID14871149.
- ^McDonald, M.R.; Fredericks, F.C.; Margerum, D.W. (1997). "Characterization of Copper(III)–Tetrapeptide Complexes with Histidine as the Third Residue".Inorganic Chemistry.36(14): 3119–3124.doi:10.1021/ic9608713.PMID11669966.
- ^Greenwood, Norman N.;Earnshaw, Alan (1997).Chemistry of the Elements(2nd ed.).Butterworth-Heinemann.p. 1187.ISBN978-0-08-037941-8.
- ^Hickman, A.; Sanford, M. (2012)."High-valent organometallic copper and palladium in catalysis".Nature.484(7393): 177–185.Bibcode:2012Natur.484..177H.doi:10.1038/nature11008.PMC4384170.PMID22498623.
- ^Liu, He; Shen, Qilong (2021). "Well-defined organometallic Copper(III) complexes: Preparation, characterization and reactivity".Coord. Chem. Rev.442:213923.doi:10.1016/j.ccr.2021.213923.
- ^A Timeline of Copper Technologies, Copper Development Association,https://www.copper.org/education/history/timeline/
- ^ab"CSA – Discovery Guides, A Brief History of Copper".Csa.com. Archived fromthe originalon 3 February 2015.Retrieved12 September2008.
- ^Rayner W. Hesse (2007).Jewelrymaking through History: an Encyclopedia.Greenwood Publishing Group. p. 56.ISBN978-0-313-33507-5.No primary source is given in that book.
- ^"Copper".Elements.vanderkrogt.net.Retrieved12 September2008.
- ^Renfrew, Colin(1990).Before civilization: the radiocarbon revolution and prehistoric Europe.Penguin.ISBN978-0-14-013642-5.Retrieved21 December2011.
- ^Thoury, M.; Mille, B.; Séverin-Fabiani, T.; Robbiola, L.; Réfrégiers, M.; Jarrige, J.-F.; Bertrand, L. (15 November 2016)."High spatial dynamics-photoluminescence imaging reveals the metallurgy of the earliest lost-wax cast object".Nature Communications.7:13356.Bibcode:2016NatCo...713356T.doi:10.1038/ncomms13356.ISSN2041-1723.PMC5116070.PMID27843139.
- ^Cowen, R."Essays on Geology, History, and People: Chapter 3: Fire and Metals".Archived fromthe originalon 10 May 2008.Retrieved7 July2009.
- ^Timberlake, S. & Prag A.J.N.W. (2005).The Archaeology of Alderley Edge: Survey, excavation and experiment in an ancient mining landscape.Oxford: John and Erica Hedges Ltd. p. 396.doi:10.30861/9781841717159.ISBN9781841717159.
- ^ab"CSA – Discovery Guides, A Brief History of Copper".CSA Discovery Guides.Archived fromthe originalon 3 February 2015.Retrieved29 April2011.
- ^abcdPompeani, David P; Steinman, Byron A; Abbott, Mark B; Pompeani, Katherine M; Reardon, William; DePasqual, Seth; Mueller, Robin H (April 2021)."On the Timing of the Old Copper Complex in North America: A Comparison of Radiocarbon Dates from Different Archaeological Contexts".Radiocarbon.63(2): 513–531.Bibcode:2021Radcb..63..513P.doi:10.1017/RDC.2021.7.ISSN0033-8222.S2CID233029733.
- ^Pleger, Thomas C. "A Brief Introduction to the Old Copper Complex of the Western Great Lakes: 4000–1000 BC",Proceedings of the Twenty-Seventh Annual Meeting of the Forest History Association of Wisconsin,Oconto, Wisconsin, 5 October 2002, pp. 10–18.
- ^Emerson, Thomas E. and McElrath, Dale L.Archaic Societies: Diversity and Complexity Across the Midcontinent,SUNY Press, 2009ISBN1-4384-2701-8.
- ^Bebber, Michelle R.; Buchanan, Briggs; Holland-Lulewicz, Jacob (26 April 2022)."Refining the chronology of North America's copper using traditions: A macroscalar approach via Bayesian modeling".PLOS ONE.17(4): e0266908.Bibcode:2022PLoSO..1766908B.doi:10.1371/journal.pone.0266908.ISSN1932-6203.PMC9041870.PMID35472064.
- ^Malakoff, David (19 March 2021)."Ancient Native Americans were among the world's first coppersmiths".Science.doi:10.1126/science.abi6135.ISSN0036-8075.S2CID233663403.
- ^abPompeani, David P.; Abbott, Mark B.; Steinman, Byron A.; Bain, Daniel J. (14 May 2013)."Lake Sediments Record Prehistoric Lead Pollution Related to Early Copper Production in North America".Environmental Science & Technology.47(11): 5545–5552.Bibcode:2013EnST...47.5545P.doi:10.1021/es304499c.ISSN0013-936X.PMID23621800.
- ^Bebber, Michelle R.; Eren, Metin I. (1 October 2018)."Toward a functional understanding of the North American Old Copper Culture" technomic devolution "".Journal of Archaeological Science.98:34–44.Bibcode:2018JArSc..98...34B.doi:10.1016/j.jas.2018.08.001.ISSN0305-4403.S2CID134060339.
- ^Dainian, Fan.Chinese Studies in the History and Philosophy of Science and Technology.p. 228.
- ^Wallach, Joel.Epigenetics: The Death of the Genetic Theory of Disease Transmission.
- ^Radivojević, Miljana; Rehren, Thilo (December 2013)."Tainted ores and the rise of tin bronzes in Eurasia, c. 6500 years ago".Antiquity Publications Ltd.
- ^abMcNeil, Ian (2002).Encyclopaedia of the History of Technology.London; New York: Routledge. pp. 13, 48–66.ISBN978-0-203-19211-5.
- ^Eastaugh, Nicholas; Walsh, Valentine; Chaplin, Tracey; Siddall, Ruth (17 June 2013).Pigment Compendium: Optical Microscopy of Historical Pigments.doi:10.4324/9780080454573.ISBN9781136373794.
- ^Rickard, T.A. (1932). "The Nomenclature of Copper and its Alloys".Journal of the Royal Anthropological Institute.62:281–290.doi:10.2307/2843960.JSTOR2843960.
- ^Timberlake, Simon (11 June 2017). "New ideas on the exploitation of copper, tin, gold, and lead ores in Bronze Age Britain: The mining, smelting, and movement of metal".Materials and Manufacturing Processes.32(7–8): 709–727.doi:10.1080/10426914.2016.1221113.S2CID138178474.
- ^Martin, Susan R. (1995)."The State of Our Knowledge About Ancient Copper Mining in Michigan".The Michigan Archaeologist.41(2–3): 119. Archived fromthe originalon 7 February 2016.
- ^abChastain, Matthew L.; Deymier-Black, Alix C.; Kelly, John E.; Brown, James A.; Dunand, David C. (1 July 2011)."Metallurgical analysis of copper artifacts from Cahokia".Journal of Archaeological Science.38(7): 1727–1736.Bibcode:2011JArSc..38.1727C.doi:10.1016/j.jas.2011.03.004.ISSN0305-4403.
- ^abCortés, Leticia Inés; Scattolin, María Cristina (June 2017)."Ancient metalworking in South America: a 3000-year-old copper mask from the Argentinian Andes".Antiquity.91(357): 688–700.doi:10.15184/aqy.2017.28.ISSN0003-598X.S2CID53068689.
- ^Hong, S.; Candelone, J.-P.; Patterson, C.C.; Boutron, C.F. (1996). "History of Ancient Copper Smelting Pollution During Roman and Medieval Times Recorded in Greenland Ice".Science.272(5259): 246–249 (247f.).Bibcode:1996Sci...272..246H.doi:10.1126/science.272.5259.246.S2CID176767223.
- ^de Callataÿ, François (2005). "The Graeco-Roman Economy in the Super Long-Run: Lead, Copper, and Shipwrecks".Journal of Roman Archaeology.18:361–372 (366–369).doi:10.1017/S104775940000742X.S2CID232346123.
- ^Savenije, Tom J.; Warman, John M.; Barentsen, Helma M.; van Dijk, Marinus; Zuilhof, Han; Sudhölter, Ernst J.R. (2000)."Corinthian Bronze and the Gold of the Alchemists"(PDF).Macromolecules.33(2): 60–66.Bibcode:2000MaMol..33...60S.doi:10.1021/ma9904870.Archived fromthe original(PDF)on 29 September 2007.
- ^Lynch, Martin (2004).Mining in World History.Reaktion Books. p. 60.ISBN978-1-86189-173-0.
- ^"Gold: prices, facts, figures and research: A brief history of money".Retrieved22 April2011.
- ^"Copper and Brass in Ships".Retrieved6 September2016.
- ^Stelter, M.; Bombach, H. (2004). "Process Optimization in Copper Electrorefining".Advanced Engineering Materials.6(7): 558–562.doi:10.1002/adem.200400403.S2CID138550311.
- ^Gardner, E. D.; et al. (1938).Copper Mining in North America.Washington, D. C.: U. S. Bureau of Mines.Retrieved19 March2019.
- ^Hyde, Charles (1998).Copper for America, the United States Copper Industry from Colonial Times to the 1990s.Tucson, Arizona: University of Arizona Press. p. passim.ISBN0-8165-1817-3.
- ^"Outokumpu Flash Smelting"(PDF).Outokumpu.p. 2. Archived fromthe original(PDF)on 24 July 2011.
- ^Karen A. Mingst (1976). "Cooperation or illusion: an examination of the intergovernmental council of copper exporting countries".International Organization.30(2): 263–287.doi:10.1017/S0020818300018270.S2CID154183817.
- ^Ryck Lydecker."Is Copper Bottom Paint Sinking?".BoatUS Magazine.Retrieved3 June2016.
- ^"Copper".American Elements.2008. Archived fromthe originalon 8 June 2008.Retrieved12 July2008.
- ^Pops, Horace, 2008, "Processing of wire from antiquity to the future",Wire Journal International,June, pp. 58–66
- ^The Metallurgy of Copper Wire,http://www.litz-wire.com/pdf%20files/Metallurgy_Copper_Wire.pdfArchived1 September 2013 at theWayback Machine
- ^Joseph, Günter, 1999, Copper: Its Trade, Manufacture, Use, and Environmental Status, edited by Kundig, Konrad J.A., ASM International, pp. 141–192 and pp. 331–375.
- ^"Copper, Chemical Element – Overview, Discovery and naming, Physical properties, Chemical properties, Occurrence in nature, Isotopes".Chemistryexplained.com.Retrieved16 October2012.
- ^Joseph, Günter, 1999, Copper: Its Trade, Manufacture, Use, and Environmental Status, edited by Kundig, Konrad J.A., ASM International, p.348
- ^"Aluminum Wiring Hazards and Pre-Purchase Inspections".www.heimer.com.Archived fromthe originalon 28 May 2016.Retrieved3 June2016.
- ^"Accelerator: Waveguides (SLAC VVC)".SLAC Virtual Visitor Center.Retrieved29 April2011.
- ^IE3 energy-saving motors, Engineer Live,http://www.engineerlive.com/Design-Engineer/Motors_and_Drives/IE3_energy-saving_motors/22687/
- ^Energy‐efficiency policy opportunities for electric motor‐driven systems, International Energy Agency, 2011 Working Paper in the Energy Efficiency Series, by Paul Waide and Conrad U. Brunner, OECD/IEA 2011
- ^Fuchsloch, J. and E.F. Brush, (2007), "Systematic Design Approach for a New Series of Ultra‐NEMA Premium Copper Rotor Motors", in EEMODS 2007 Conference Proceedings, 10–15 June, Beijing.
- ^Copper motor rotor project; Copper Development Association;"Copper.org: Copper Motor Rotor Project".Archived fromthe originalon 13 March 2012.Retrieved7 November2012.
- ^NEMA Premium Motors, The Association of Electrical Equipment and Medical Imaging Manufacturers;"NEMA - NEMA Premium Motors".Archived fromthe originalon 2 April 2010.Retrieved12 October2009.
- ^"Renewables 2022 – Analysis".IEA.Retrieved16 August2023.
- ^"REN21 Renewables Global Status Report".REN21.14 June 2019.Retrieved16 August2023.
- ^Will the Transition to Renewable Energy Be Paved in Copper?,Renewable Energy World;Jan 15, 2016;https://www.renewableenergyworld.com/articles/2016/01/will-the-transition-to-renewable-energy-be-paved-in-copper.htmlArchived2018-06-22 at theWayback Machine
- ^García-Olivares, Antonio, Joaquim Ballabrera-Poy, Emili García-Ladona, and Antonio Turiel. A global renewable mix with proven technologies and common materials, Energy Policy, 41 (2012): 561-57,http://imedea.uib-csic.es/master/cambioglobal/Modulo_I_cod101601/Ballabrera_Diciembre_2011/Articulos/Garcia-Olivares.2011.pdf
- ^"Energy saving - A kilo more of copper increases environmental performance by 100 to 1,000 times".Renewable Energy Magazine, at the heart of clean energy journalism.14 April 2011.Retrieved16 August2023.
- ^Copper at the core of renewable energies; European Copper Institute; European Copper Institute; 18 pages;http://www.eurocopper.org/files/presskit/press_kit_copper_in_renewables_final_29_10_2008.pdfArchived2012-05-23 at theWayback Machine
- ^Copper in energy systems; Copper Development Association Inc.;http://www.copper.org/environment/green/energy.htmlArchived2020-08-01 at theWayback Machine
- ^The Rise Of Solar: A Unique Opportunity For Copper; Solar Industry Magazine; April 2017; Zolaika Strong;https://issues.solarindustrymag.com/article/rise-solar-unique-opportunity-copperArchived2022-10-30 at theWayback Machine
- ^Pops, Horace, 1995. Physical Metallurgy of Electrical Conductors, in Nonferrous Wire Handbook, Volume 3: Principles and Practice, The Wire Association International
- ^The World Copper Factbook, 2017;http://www.icsg.org/index.php/component/jdownloads/finish/170/2462
- ^Copper Mineral Commodity Summary (USGS, 2017)https://minerals.usgs.gov/minerals/pubs/commodity/copper/mcs-2017-coppe.pdf
- ^Global Mineral Resource Assessment (USGS, 2014)http://pubs.usgs.gov/fs/2014/3004/pdf/fs2014-3004.pdf
- ^Long-Term Availability of Copper; International Copper Association;http://copperalliance.org/wordpress/wp-content/uploads/2018/02/ICA-long-term-availability-201802-A4-HR.pdfArchived2018-06-29 at theWayback Machine
- ^Will the Transition to Renewable Energy Be Paved in Copper?, Renewable Energy World; Jan 15, 2016; by Zolaikha Strong;https://www.renewableenergyworld.com/articles/2016/01/will-the-transition-to-renewable-energy-be-paved-in-copper.htmlArchived2018-06-22 at theWayback Machine
- ^Seale, Wayne (2007). The role of copper, brass, and bronze in architecture and design;Metal Architecture,May 2007
- ^Copper roofing in detail; Copper in Architecture; Copper Development Association, U.K., www.cda.org.uk/arch
- ^Architecture, European Copper Institute;http://eurocopper.org/copper/copper-architecture.htmlArchived9 October 2012 at theWayback Machine
- ^Kronborg completed; Agency for Palaces and Cultural Properties, København,"Kronborg completed - Agency for Palaces and Cultural Properties".Archived fromthe originalon 24 October 2012.Retrieved12 September2012.
- ^Berg, Jan."Why did we paint the library's roof?".Archived fromthe originalon 25 June 2007.Retrieved20 September2007.
- ^Architectural considerations; Copper in Architecture Design Handbook,http://www.copper.org/applications/architecture/arch_dhb/fundamentals/arch_considerations.htm[permanent dead link]
- ^Peters, Larry E. (2004). Preventing corrosion on copper roofing systems; Professional Roofing, October 2004,http://www.professionalroofing.net
- ^Wu, Chun."Oxidation reaction: Why is the Statue of Liberty blue-green? How does rust work?"(PDF).wepanknowledgecenter.org.Engage Engineering. Archived fromthe original(PDF)on 25 October 2013.Retrieved25 October2013.
- ^Fitzgerald, K.P.; Nairn, J.; Atrens, A. (1998). "The chemistry of copper patination".Corrosion Science.40(12): 2029–50.Bibcode:1998Corro..40.2029F.doi:10.1016/S0010-938X(98)00093-6.
- ^Application Areas: Architecture – Finishes – patina;http://www.copper.org/applications/architecture/finishes.html
- ^Glossary of copper terms, Copper Development Association (UK):"Glossary of copper terms".Archived fromthe originalon 20 August 2012.Retrieved14 September2012.
- ^Finishes – natural weathering; Copper in Architecture Design Handbook, Copper Development Association Inc.,"Copper.org: Architecture Design Handbook: Finishes".Archived fromthe originalon 16 October 2012.Retrieved12 September2012.
- ^Davis, Joseph R. (2001).Copper and Copper Alloys.ASM International. pp. 3–6, 266.ISBN978-0-87170-726-0.
- ^Edding, Mario E., Flores, Hector, and Miranda, Claudio, (1995), Experimental Usage of Copper-Nickel Alloy Mesh in Mariculture. Part 1: Feasibility of usage in a temperate zone; Part 2: Demonstration of usage in a cold zone; Final report to the International Copper Association Ltd.
- ^Corrosion Behaviour of Copper Alloys used in Marine AquacultureArchived24 September 2013 at theWayback Machine.(PDF). copper.org. Retrieved on 8 November 2011.
- ^Copper Touch SurfacesArchived23 July 2012 at theWayback Machine.Copper Touch Surfaces. Retrieved on 8 November 2011.
- ^"EPA Registers Copper Surfaces for Residual Use Against Coronavirus".United States Environmental Protection Agency.10 February 2021.Retrieved11 October2021.
- ^Montero, David A.; Arellano, Carolina; Pardo, Mirka; Vera, Rosa; Gálvez, Ricardo; Cifuentes, Marcela; Berasain, María A.; Gómez, Marisol; Ramírez, Claudio; Vidal, Roberto M. (5 January 2019)."Antimicrobial properties of a novel copper-based composite coating with potential for use in healthcare facilities".Antimicrobial Resistance and Infection Control.8(1): 3.doi:10.1186/s13756-018-0456-4.ISSN2047-2994.PMC6321648.PMID30627427.
- ^ab"EPA registers copper-containing alloy products".United States Environmental Protection Agency.May 2008. Archived fromthe originalon 29 September 2015.
- ^Biurrun, Amaya; Caballero, Luis; Pelaz, Carmen; León, Elena; Gago, Alberto (1999)."Treatment of a Legionella pneumophila-Colonized Water Distribution System Using Copper-Silver Ionization and Continuous Chlorination"(PDF).Infection Control and Hospital Epidemiology.20(6): 426–428.doi:10.1086/501645.JSTOR30141645.PMID10395146.S2CID32388649.Archived fromthe original(PDF)on 17 February 2019.
- ^Zaleski, Andrew,As hospitals look to prevent infections, a chorus of researchers make a case for copper surfaces,STAT, 24 September 2020
- ^Chilean subway protected with Antimicrobial Copper – Rail News fromArchived24 July 2012 at theWayback Machine.rail.co. Retrieved on 8 November 2011.
- ^Codelco to provide antimicrobial copper for new metro lines (Chile)[dead link].Construpages.com.ve. Retrieved on 8 November 2011.
- ^PR 811 Chilean Subway Installs Antimicrobial CopperArchived23 November 2011 at theWayback Machine.(PDF). antimicrobialcopper.com. Retrieved on 8 November 2011.
- ^"Copper and Cupron".Cupron.
- ^GlobalData (17 November 2023)."Global copper supply in 2023 will be supported by increased output from the DRC, Peru, and Chile".Mining Technology.Retrieved22 December2023.
- ^Woods, Bob (27 September 2023)."Copper is critical to energy transition. The world is falling way behind on producing enough".CNBC.Retrieved22 December2023.
- ^"China drives copper to 4-month low, raising global economic alarms".Nikkei Asia.Retrieved22 December2023.
- ^"Repairing aluminum wiring"(PDF).U.S. Consumer Product Safety Commission.p. 1. Archived fromthe original(PDF)on 25 December 2016.Retrieved23 December2023.
A national survey conducted by Franklin Research Institute for CPSC showed that homes built before 1972, and wired with aluminum, are 55 times more likely to have one or more wire connections at outlets reach "Fire Hazard Conditions" than homes wired with copper.
- ^Hellemans, Alexander (1 January 2007)."Manufacturing Mayday: Production glitches send Airbus into a tailspin".IEEE Spectrum.Retrieved19 June2014.
- ^"Global copper market under supplied, demand on the rise – report".Mining.com.6 January 2019.Retrieved13 January2019.
- ^"Will the Transition to Renewable Energy Be Paved in Copper?".www.renewableenergyworld.com.15 January 2015. Archived fromthe originalon 22 June 2018.Retrieved13 January2019.
- ^"Copper and cars: Boom goes beyond electric vehicles".MINING.com.18 June 2018.Retrieved13 January2019.
- ^"Impact of electric cars in medium-term copper demand 'overrated', experts say".MINING.com.12 April 2018.Retrieved13 January2019.
- ^"Why are Premiums for Copper Bullion So High?".Provident Metals.20 August 2012.Retrieved23 January2019.
- ^Chace, Zoe."Penny Hoarders Hope for the Day The Penny Dies".NPR.org.NPR.Retrieved23 January2019.
- ^Walker, W.R.; Keats, D.M. (1976). "An investigation of the therapeutic value of the 'copper bracelet'-dermal assimilation of copper in arthritic/rheumatoid conditions".Agents and Actions.6(4): 454–459.PMID961545.
- ^Richmond SJ, Gunadasa S, Bland M, Macpherson H (2013)."Copper bracelets and magnetic wrist straps for rheumatoid arthritis – analgesic and anti-inflammatory effects: a randomised double-blind placebo controlled crossover trial".PLOS ONE.8(9): e71529.Bibcode:2013PLoSO...871529R.doi:10.1371/journal.pone.0071529.PMC3774818.PMID24066023.
- ^Richmond, Stewart J.; Brown, Sally R.; Campion, Peter D.; Porter, Amanda J.L.; Moffett, Jennifer A. Klaber; Jackson, David A.; Featherstone, Valerie A.; Taylor, Andrew J. (2009)."Therapeutic effects of magnetic and copper bracelets in osteoarthritis: A randomised placebo-controlled crossover trial".Complementary Therapies in Medicine.17(5–6): 249–256.doi:10.1016/j.ctim.2009.07.002.ISSN0965-2299.PMID19942103.
- ^"Find the Truth Behind Medical Myths".University of Arkansas for Medical Sciences. 6 January 2014. Archived fromthe originalon 6 January 2014.
While it's never been proven that copper can be absorbed through the skin by wearing a bracelet, research has shown that excessive copper can result in poisoning, causing vomiting and, in severe cases, liver damage.
- ^abGeoffrey Michael Gadd(March 2010)."Metals, minerals and microbes: geomicrobiology and bioremediation".Microbiology.156(3): 609–643.doi:10.1099/mic.0.037143-0.PMID20019082.
- ^Harbhajan Singh (2006).Mycoremediation: Fungal Bioremediation.John Wiley & Sons. p. 509.ISBN978-0-470-05058-3.
- ^Vest, Katherine E.; Hashemi, Hayaa F.; Cobine, Paul A. (2013). "The Copper Metallome in Eukaryotic Cells". In Banci, Lucia (ed.).Metallomics and the Cell.Metal Ions in Life Sciences. Vol. 12. Springer. pp. 451–78.doi:10.1007/978-94-007-5561-1_13.ISBN978-94-007-5560-4.PMID23595680.electronic-bookISBN978-94-007-5561-1ISSN1559-0836electronic-ISSN1868-0402
- ^"Fun facts".Horseshoe crab.University of Delaware. Archived fromthe originalon 22 October 2008.Retrieved13 July2008.
- ^abS.J. Lippard, J.M. Berg "Principles of bioinorganic chemistry" University Science Books: Mill Valley, CA; 1994.ISBN0-935702-73-3.
- ^Decker, H. & Terwilliger, N. (2000)."COPs and Robbers: Putative evolution of copper oxygen-binding proteins".Journal of Experimental Biology.203(Pt 12): 1777–1782.doi:10.1242/jeb.203.12.1777.PMID10821735.
- ^ Schneider, Lisa K.; Wüst, Anja; Pomowski, Anja; Zhang, Lin; Einsle, Oliver (2014). "No Laughing Matter: The Unmaking of the Greenhouse Gas Dinitrogen Monoxide by Nitrous Oxide Reductase". In Peter M.H. Kroneck; Martha E. Sosa Torres (eds.).The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment.Metal Ions in Life Sciences. Vol. 14. Springer. pp. 177–210.doi:10.1007/978-94-017-9269-1_8.ISBN978-94-017-9268-4.PMID25416395.
- ^Denoyer, Delphine; Clatworthy, Sharnel A.S.; Cater, Michael A. (2018). "Chapter 16. Copper Complexes in Cancer Therapy". In Sigel, Astrid; Sigel, Helmut; Freisinger, Eva; Sigel, Roland K.O. (eds.).Metallo-Drugs: Development and Action of Anticancer Agents.Vol. 18. Berlin: de Gruyter GmbH. pp. 469–506.doi:10.1515/9783110470734-016.ISBN978-3-11-047073-4.PMID29394035.
{{cite book}}:|journal=ignored (help) - ^ab"Amount of copper in the normal human body, and other nutritional copper facts".Archived fromthe originalon 10 April 2009.Retrieved3 April2009.
- ^Adelstein, S. J.; Vallee, B. L. (1961). "Copper metabolism in man".New England Journal of Medicine.265(18): 892–897.doi:10.1056/NEJM196111022651806.PMID13859394.
- ^M.C. Linder; Wooten, L.; Cerveza, P.; Cotton, S.; Shulze, R.; Lomeli, N. (1 May 1998)."Copper transport".The American Journal of Clinical Nutrition.67(5): 965S–971S.doi:10.1093/ajcn/67.5.965S.PMID9587137.
- ^Frieden, E.; Hsieh, H.S. (1976).Ceruloplasmin: The copper transport protein with essential oxidase activity.Advances in Enzymology – and Related Areas of Molecular Biology. Vol. 44. pp. 187–236.doi:10.1002/9780470122891.ch6.ISBN978-0-470-12289-1.JSTOR20170553.PMID775938.
{{cite book}}:|journal=ignored (help) - ^S.S. Percival; Harris, E.D. (1 January 1990). "Copper transport from ceruloplasmin: Characterization of the cellular uptake mechanism".American Journal of Physiology. Cell Physiology.258(1): C140–C146.doi:10.1152/ajpcell.1990.258.1.c140.PMID2301561.
- ^Dietary Reference Intakes: RDA and AI for Vitamins and ElementsArchived13 November 2018 at theWayback MachineFood and Nutrition Board, Institute of Medicine, National Academies Press, 2011. Retrieved 18 April 2018.
- ^Copper. IN:Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Copper.National Academy Press. 2001, PP. 224–257.
- ^"Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies"(PDF).2017.
- ^Tolerable Upper Intake Levels For Vitamins And Minerals(PDF),European Food Safety Authority, 2006
- ^"Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR p. 33982"(PDF).
- ^"Daily Value Reference of the Dietary Supplement Label Database (DSLD)".Dietary Supplement Label Database (DSLD).Archived fromthe originalon 7 April 2020.Retrieved16 May2020.
- ^Bonham, Maxine; O'Connor, Jacqueline M.; Hannigan, Bernadette M.; Strain, J.J. (2002)."The immune system as a physiological indicator of marginal copper status?".British Journal of Nutrition.87(5): 393–403.doi:10.1079/BJN2002558.PMID12010579.
- ^Li, Yunbo; Trush, Michael; Yager, James (1994). "DNA damage caused by reactive oxygen species originating from a copper-dependent oxidation of the 2-hydroxy catechol of estradiol".Carcinogenesis.15(7): 1421–1427.doi:10.1093/carcin/15.7.1421.PMID8033320.
- ^Gordon, Starkebaum; John, M. Harlan (April 1986)."Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine".J. Clin. Invest.77(4): 1370–6.doi:10.1172/JCI112442.PMC424498.PMID3514679.
- ^"Pesticide Information Profile for Copper Sulfate".Cornell University.Retrieved10 July2008.
- ^Hunt, Charles E. & William W. Carlton (1965). "Cardiovascular Lesions Associated with Experimental Copper Deficiency in the Rabbit".Journal of Nutrition.87(4): 385–394.doi:10.1093/jn/87.4.385.PMID5841854.
- ^Ayyat M.S.; Marai I.F.M.; Alazab A.M. (1995)."Copper-Protein Nutrition of New Zealand White Rabbits under Egyptian Conditions".World Rabbit Science.3(3): 113–118.doi:10.4995/wrs.1995.249.hdl:10251/10503.
- ^Brewer GJ (March 2012). "Copper excess, zinc deficiency, and cognition loss in Alzheimer's disease".BioFactors(Review).38(2): 107–113.doi:10.1002/biof.1005.hdl:2027.42/90519.PMID22438177.S2CID16989047.
- ^"Copper: Alzheimer's Disease".Examine.com.Retrieved21 June2015.
- ^NIOSH Pocket Guide to Chemical Hazards."#0151".National Institute for Occupational Safety and Health(NIOSH).
- ^NIOSH Pocket Guide to Chemical Hazards."#0150".National Institute for Occupational Safety and Health(NIOSH).
- ^OEHHACopper
- ^Talhout, Reinskje; Schulz, Thomas; Florek, Ewa; Van Benthem, Jan; Wester, Piet; Opperhuizen, Antoon (2011)."Hazardous Compounds in Tobacco Smoke".International Journal of Environmental Research and Public Health.8(12): 613–628.doi:10.3390/ijerph8020613.ISSN1660-4601.PMC3084482.PMID21556207.
- ^Pourkhabbaz, A.; Pourkhabbaz, H. (2012)."Investigation of Toxic Metals in the Tobacco of Different Iranian Cigarette Brands and Related Health Issues".Iranian Journal of Basic Medical Sciences.15(1): 636–644.PMC3586865.PMID23493960.
- ^Bernhard, David; Rossmann, Andrea; Wick, Georg (2005)."Metals in cigarette smoke".IUBMB Life.57(12): 805–809.doi:10.1080/15216540500459667.PMID16393783.S2CID35694266.
Notes
 |
 |
 |
 |
| in pure water, or acidic or alkali conditions. Copper in neutral water is more noble than hydrogen. | in water containing sulfide | in 10 M ammonia solution | in a chloride solution |
Further reading
- Massaro, Edward J., ed. (2002).Handbook of Copper Pharmacology and Toxicology.Humana Press.ISBN978-0-89603-943-8.
- "Copper: Technology & Competitiveness (Summary)Chapter 6: Copper Production Technology "(PDF).Office of Technology Assessment. 2005.
- Current Medicinal Chemistry, Volume 12, Number 10, May 2005, pp. 1161–1208(48) Metals, Toxicity and Oxidative Stress
- William D. Callister (2003).Materials Science and Engineering: an Introduction(6th ed.). Wiley, New York. Table 6.1, p. 137.ISBN978-0-471-73696-7.
- Material: Copper (Cu), bulk,MEMS and Nanotechnology Clearinghouse.
- Kim BE; Nevitt T; Thiele DJ (2008). "Mechanisms for copper acquisition, distribution and regulation".Nat. Chem. Biol.4(3): 176–85.doi:10.1038/nchembio.72.PMID18277979.
External links
- CopperatThe Periodic Table of Videos(University of Nottingham)
- Copper and compounds fact sheetfrom theNational Pollutant Inventoryof Australia
- International Copper Association and the Copper Alliance,a business interest group
- Copper.org– official website of the Copper Development Association, a North American industry association with an extensive site of properties and uses of copper
- Price historyofLME Copper,according to the IMF


