Cristobalite
| Cristobalite | |
|---|---|
 Cristobalitespherulitesformed bydevitrificationfrom theobsidianmatrix. Specimen from California, US; size: 5.9 cm × 3.8 cm × 3.8 cm (2.3 in × 1.5 in × 1.5 in). | |
| General | |
| Category | Oxide mineral,quartzgroup |
| Formula (repeating unit) | SiO2 |
| IMA symbol | Crs[1] |
| Strunz classification | 4.DA.15 |
| Dana classification | 75.1.1.1 |
| Crystal system | Tetragonal |
| Crystal class | Trapezohedral(422) |
| Space group | P41212,P43212 |
| Unit cell | a= 4.9709(1) Å, c= 6.9278(2) Å; Z= 4 (α polytype) |
| Structure | |
| Jmol(3D) | Interactive image |
| Identification | |
| Color | Colorless, white |
| Crystal habit | Octahedraorspherulitesup to several cm in diameter |
| Twinning | on {111} |
| Fracture | Conchoidal |
| Tenacity | Brittle |
| Mohs scalehardness | 6–7 |
| Luster | Vitreous |
| Streak | White |
| Diaphaneity | Transparent |
| Specific gravity | 2.32–2.36 |
| Optical properties | Uniaxial (−) |
| Refractive index | nω= 1.487 nε= 1.484 |
| Birefringence | 0.003 |
| Pleochroism | None |
| Melting point | 1,713 °C (3,115 °F) (β)[2] |
| References | [3][4][5][6] |
Cristobalite(/krɪˈstoʊbəˌlaɪt/) is amineralpolymorphofsilicathat is formed at very high temperatures. It has the same chemical formula asquartz,SiO2,but a distinct crystal structure. Both quartz and cristobalite are polymorphs with all the members of the quartz group, which also includecoesite,tridymiteandstishovite.It is named after Cerro San Cristóbal inPachuca Municipality,Hidalgo,Mexico.
It is used in dentistry as a component ofalginateimpression materials as well as for making models of teeth.[7]
Properties
[edit]Metastability
[edit]Cristobalite is stable only above 1470 °C, but can crystallize and persistmetastablyat lower temperatures. The persistence of cristobalite outside its thermodynamic stability range occurs because the transition from cristobalite toquartzortridymiteis "reconstructive", requiring the breaking up and reforming of thesilicaframework. These frameworks are composed ofSiO4tetrahedrain which every oxygen atom is shared with a neighbouring tetrahedron, so that thechemical formulaof silica is SiO2.The breaking of these bonds, required to convert cristobalite to tridymite and quartz, requires considerableactivation energyand may not happen on a human time frame at room temperature. Framework silicates are also known astectosilicates.
Whendevitrifyingsilica, cristobalite is usually the first phase to form, even when well outside its thermodynamic stability range. This is an example ofOstwald's step rule.The dynamically disordered nature of the β phase is partly responsible for the low enthalpy of fusion of silica.
Structures
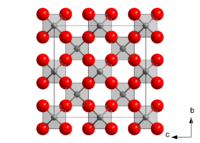
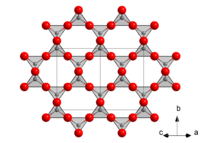
[edit]There is more than one form of the cristobalite framework. At high temperatures, the structure is called β-cristobalite. It is in thecubic crystal system,space groupFd3m (No. 227,Pearson symbolcF104).[8]It has thediamond structurebut with linked tetrahedra of silicon and oxygen where the carbon atoms are in diamond. Achiraltetragonalform called α-cristobalite (space group either P41212, No. 92,[9]or P43212, No. 96, at random) occurs on cooling below about 250 °C at ambient pressure and is related to the cubic form by static tilting of the silica tetrahedra in the framework. This transition is variously called the low-high ortransition. It may be termed "displacive"; i.e., it is not generally possible to prevent the cubic β form from becoming tetragonal by rapid cooling. Under rare circumstances the cubic form may be preserved if the crystal grain is pinned in a matrix that does not allow for the considerable spontaneous strain that is involved in the transition, which causes a change in shape of the crystal. This transition is highly discontinuous. Going from the α form to the β form causes an increase in volume of 3[10]or 4[11]percent. The exact transition temperature depends on the crystallinity of the cristobalite sample, which itself depends on factors such as how long it has been annealed at a particular temperature.
The cubic β phase consists of dynamically disordered silica tetrahedra. The tetrahedra remain fairly regular and are displaced from their ideal static orientations due to the action of a class of low-frequencyphononscalledrigid unit modes.It is the "freezing" of one of these rigid unit modes that is the soft mode for the α–β transition.
In β-cristobalite, there are right-handed and left-handed helices of tetrahedra (or of silicon atoms) parallel to all three axes. In the α–β phase transition, however, only the right-handed or the left-handed helix in one direction is preserved (the other becoming a two-fold screw axis), so only one of the three degenerate cubic crystallographic axes retains a fourfold rotational axis (actually ascrew axis) in the tetragonal form. (That axis becomes the "c" axis, and the new "a" axes are rotated 45° compared to the other two old axes. The new "a" lattice parameter is shorter by approximately the square root of 2, so the α unit cell contains only 4 silicon atoms rather than 8.) The choice of axis is arbitrary, so that varioustwinscan form within the same grain. These different twin orientations coupled with the discontinuous nature of the transition (volume and slight shape change) can cause considerable mechanical damage to materials in which cristobalite is present and that pass repeatedly through the transition temperature, such as refractory bricks.
-
An idealized model of β-cristobalite, showing corner-bondedSiO4tetrahedra. There is a right-handed four-fold screw axis at the centre of half the white squares, and a left-handed one at the centre of the others. In this projection we see glide planes parallel to the axes and mirrors on the diagonals. In reality the tetrahedra are constantly wobbling.
-
β-cristobalite viewed along the 101 direction
-
The crumpled framework of α-cristobalite, related to the β form by static tilting of the tetrahedra. This view corresponds to the view along the 101 direction of the previous illustration, except that the "b" axis of that picture is now horizontal. The two-fold screw axes appear here as two-fold axes of rotation going through the middle of the white areas and between the pairs of almost superimposed oxygen atoms.
-
Unit cell of α-cristobalite; red spheres are oxygen atoms. We see here five silicon atoms in a helix (the first and the last are equivalent atoms in the lattice) going in the "c" direction (into the page). The horizontal and vertical axes are the "a" axes.
-
Unit cell of β-cristobalite; red spheres are oxygen atoms.
Occurrence
[edit]Cristobalite occurs as white octahedra orspherulitesin acidic volcanic rocks and in converted diatomaceous deposits in theMonterey Formationof the US state of California and similar areas.
The micrometre-scale spheres that make up preciousopalexhibit some X-ray diffraction patterns that are similar to that of cristobalite, but lack any long-range order so they are not considered true cristobalite. In addition, the presence of structural water in opal makes it doubtful that opal consists of cristobalite.[12][13]
Cristobalite is visible as the white inclusions insnowflake obsidian,a volcanic glass.
References
[edit]- ^Warr, L. N. (2021)."IMA–CNMNC approved mineral symbols".Mineralogical Magazine.85(3): 291–320.Bibcode:2021MinM...85..291W.doi:10.1180/mgm.2021.43.S2CID235729616.
- ^Deer, W. A.; Howie, R. A.; Zussman, J. (1966).An Introduction to the Rock Forming Minerals.Longman.pp. 340–355.ISBN0-582-44210-9.
- ^Mineralienatlas.
- ^CristobaliteArchived2010-07-15 at theWayback Machine.Handbook of Mineralogy.
- ^Cristobalite.Mindat.
- ^"Cristobalite Mineral Data".Webmineral.
- ^Anusavice, Kenneth J. (2013).Phillips' science of dental materials.Elsevier/Saunders.ISBN978-1-4377-2418-9.OCLC934359978.
- ^Wright A. F., Leadbetter A. J. (1975). "The structures of the b-cristobalite phases of SiO2and AlPO4".Philosophical Magazine.31(6): 1391–1401.Bibcode:1975PMag...31.1391W.doi:10.1080/00318087508228690.
- ^Downs R. T., Palmer D. C. (1994)."The pressure behavior of a cristobalite"(PDF).American Mineralogist.79:9–14. Archived fromthe original(PDF)on 2019-05-15.Retrieved2009-12-15.
- ^R.E. Smallman & R.J. Bishop (1999). "2".Modern Physical Metallurgy and Materials Engineering(6 ed.). Butterworth-Heinemann.ISBN978-0-7506-4564-5.
- ^A.J. Leadbetter & A.F. Wright (1976). "The α—β transition in the cristobalite phases of SiO2and AIPO4I. X-ray studies ".The Philosophical Magazine.33(1): 105–112.Bibcode:1976PMag...33..105L.doi:10.1080/14786437608221095.
- ^Deane K. Smith (1998)."Opal, cristobalite, and tridymite: Noncrystallinity versus crystallinity, nomenclature of the silica minerals and bibliography".Powder Diffraction.13(1): 2–19.Bibcode:1998PDiff..13....2S.doi:10.1017/S0885715600009696.S2CID97394861.
- ^"Silica, Crystalline - Overview | Occupational Safety and Health Administration"(PDF).www.osha.gov.Archived fromthe original(PDF)on 2016-03-04.
Further reading
[edit]- American Geological Institute Dictionary of Geological Terms.
- Durham, D. L., "Monterey Formation: Diagenesis". in:Uranium in the Monterey Formation of California.US Geological Survey Bulletin 1581-A, 1987.
- Reviews in Mineralogy and Geochemistry,vol. 29.,Silica: behavior, geochemistry and physical applications.Mineralogical Society of America, 1994.
- R. B. Sosman,The Phases of Silica.(Rutgers University Press, 1965)