Cyclic peptide
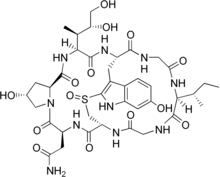


Cyclic peptidesarepolypeptide chainswhich contain a circular sequence of bonds.[1]This can be through a connection between the amino and carboxyl ends of the peptide, for example incyclosporin;a connection between the amino end and a side chain, for example inbacitracin;the carboxyl end and a side chain, for example incolistin;or two side chains or more complicated arrangements, for example inalpha-amanitin.Many cyclic peptides have been discovered in nature and many others have been synthesized in the laboratory. Their length ranges from just two amino acid residues to hundreds. In nature they are frequently antimicrobial or toxic; in medicine they have various applications, for example as antibiotics and immunosuppressive agents.[2] Thin-Layer Chromatography(TLC) is a convenient method to detect cyclic peptides in crude extract from bio-mass.[3]
Classification
[edit]Cyclic peptides can be classified according to the types of bonds that comprise the ring.
- Homodetic cyclic peptides, such as cyclosporine A, are those in which the ring is composed exclusively of normal peptide bonds (i.e. between the alpha carboxyl of one residue to the alpha amine of another). The smallest such species are2,5-diketopiperazines,[4]being derived from the cyclisation of a dipeptide.
- Cyclic isopeptides contain at least one non-alpha amide linkage, such as a linkage between the side chain of one residue to the alpha carboxyl group of another residue, as in microcystin and bacitracin.
- Cyclicdepsipeptides,such asaureobasidin Aand HUN-7293, have at least one lactone (ester) linkage in place of one of the amides. Some cyclic depsipeptides are cyclized between the C-terminal carboxyl and the side chain of a Thr or Ser residue in the chain, such as kahalalide F, theonellapeptolide, anddidemninB.
- Bicyclics such as theamanitinsand thephalloidinscontain a bridging group, generally between two of the side chains. In the amatoxins, this is formed as asulfoxidebridge between the Trp and Cys residues. Other bicyclic peptides include echinomycin, triostin A, and Celogentin C.
- There are a number of bi and monocyclic peptides which are cyclized through adisulfide bondbetween twocysteines,oxytocinbeing a notable example.
Biosynthesis
[edit]Cyclic peptides in plants are synthesized via a two-step process; thetranslationof a linear peptide chain, and its subsequent formation into a cyclic structure through activities of a protease-like enzyme or other ways.[5][6][7]
Properties and applications
[edit]Cyclic peptides tend to be extremely resistant to the process of digestion, making them of interest to scientists working on novel oral medications.[8]
Examples include:
See also
[edit]- Nonribosomal peptide
- lantibiotics,19-37 residues and 1 to 5 'bridges'
References
[edit]- ^Salehi, David; Mozaffari, Saghar; Zoghebi, Khalid; Lohan, Sandeep; Mandal, Dindyal; Tiwari, Rakesh K.; Parang, Keykavous (2022-03-29)."Amphiphilic Cell-Penetrating Peptides Containing Natural and Unnatural Amino Acids as Drug Delivery Agents".Cells.11(7): 1156.doi:10.3390/cells11071156.ISSN2073-4409.PMC8997995.PMID35406720.
- ^Jensen, Knud (2009-09-01).Peptide and Protein Design for Biopharmaceutical Applications.John Wiley & Sons.ISBN9780470749715.
- ^Wenyan, Xu; Jun, Tang; Changjiu, Ji; Wenjun, He; Ninghua, Tan (2008). "Application of a TLC chemical method to detection of cyclotides in plants".Science Bulletin.53(11): 1671–1674.Bibcode:2008SciBu..53.1671W.doi:10.1007/s11434-008-0178-8.
- ^Borthwick AD (May 2012). "2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products".Chemical Reviews.112(7): 3641–3716.doi:10.1021/cr200398y.PMID22575049.
- ^Barber, Carla J. S.; Pujara, Pareshkumar T.; Reed, Darwin W.; Chiwocha, Shiela; Zhang, Haixia; Covello, Patrick S. (2013)."The Two-step Biosynthesis of Cyclic Peptides from Linear Precursors in a Member of the Plant Family Caryophyllaceae Involves Cyclization by a Serine Protease-like Enzyme".Journal of Biological Chemistry.288(18): 12500–12510.doi:10.1074/jbc.M112.437947.PMC3642298.PMID23486480.
- ^Wenyan Xu; et al. (2011)."Various mechanisms in cyclopeptide production from precursors synthesized independently of non-ribosomal peptide synthetases".Acta Biochimica et Biophysica Sinica.43(10): 757–762.doi:10.1093/abbs/gmr062.PMC3180235.PMID21764803.
- ^Wenyan Xu; et al."Plant Cyclopeptides and Possible Biosynthetic Mechanisms".
{{cite journal}}:Cite journal requires|journal=(help) - ^David J. Craik (17 March 2006). "Seamless Proteins Tie Up Their Loose Ends".Science.311(5767): 1563–7.doi:10.1126/science.1125248.PMID16543448.S2CID82425866.
External links
[edit]- Cybase
- Cyclic+Peptidesat the U.S. National Library of MedicineMedical Subject Headings(MeSH)
