Eosin
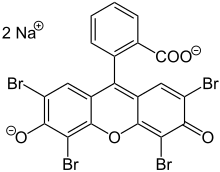
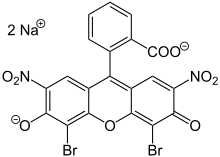
Eosinis the name of severalfluorescentacidic compounds which bind to and form salts with basic, oreosinophilic,compounds like proteins containing amino acid residues such asarginineandlysine,and stains them dark red or pink as a result of the actions ofbromineon eosin. In addition to staining proteins in thecytoplasm,it can be used to staincollagenandmuscle fibersfor examination under themicroscope.Structures that stain readily with eosin are termed eosinophilic. In the field ofhistology,Eosin Yis the form of eosin used most often as ahistologic stain.[1][2]
Etymology[edit]
Eosin was named by its inventorHeinrich Caroafter the nickname (Eos) of a childhood friend, Anna Peters.[3]
Variants[edit]

There are actually two very closely related compounds commonly referred to as eosin. Most often used is inhistologyisEosin Y[1][2](also known aseosin Y ws,eosin yellowish,Acid Red 87,C.I. 45380,bromoeosine,bromofluoresceic acid,D&C Red No. 22); it has a very slightly yellowish cast. The other eosin compound isEosin B(eosin bluish,Acid Red 91,C.I. 45400,Saffrosine,Eosin Scarlet,orimperial red); it has a very faint bluish cast. The two dyes are interchangeable, and the use of one or the other is a matter of preference and tradition.
Eosin Y is a tetrabromo derivative offluorescein.[4]Eosin B is a dibromo dinitro derivative offluorescein.[5]
Uses[edit]
Use in histology[edit]

Eosin is most often used as acounterstaintohematoxylininH&E (haematoxylin and eosin) staining.H&E staining is one of the most commonly used techniques inhistology.Tissuestained withhaematoxylinand eosin showscytoplasmstained pink-orange andnucleistained darkly, either blue or purple. Eosin also stainsred blood cellsintensely red.
For staining, eosin Y is typically used in concentrations of 1 to 5 percent weight by volume, dissolved in water orethanol.[6]For prevention of mold growth in aqueous solutions,thymolis sometimes added.[7]A small concentration (0.5 percent) ofacetic acidusually gives a deeper red stain to the tissue.
It is listed as anIARCclass 3 carcinogen.
Other uses[edit]

Eosin is also used as a red dye in inks; however, the molecule, especially that of eosin Y, tends to degrade over time, leaving behind its bromine atoms, hence causing paint incorporating such a dye to obtain a darker brown tinge over time.[8]A notable user of eosin dye was thepost-impressionistpainterVan Gogh.
See also[edit]
References[edit]
- ^abLillie, Ralph Dougall (1977).H. J. Conn's Biological stains(9th ed.). Baltimore: Williams & Wilkins. pp. 692p.
- ^abBancroft, John; Stevens, Alan, eds. (1982).The Theory and Practice of Histological Techniques(2nd ed.). Longman Group Limited.
- ^Travis, Anthony S (1998). ""Ambitious and Glory Hunting... Impractical and Fantastic": Heinrich Caro at BASF ".Technology and Culture.39(1): 105–115.doi:10.2307/3107005.JSTOR3107005.
- ^ItsCAS numberisand itsSMILESstructure isO=C5C(Br)=C2O C1=C(Br)C([O-]) =C(Br)C=C1C(C4=C (C([O-])=O)C=C C=C4)=C2C=C3Br.
- ^ItsCAS numberisand itsSMILESstructure isO=C5C(Br)=C2O C1=C(Br)C([O-]) =C([N+]([O-])=O) C=C1C(C4=C(C([O-]) =O)C=CC=C4)=C2 C=C3[N+]([O-])=O.
- ^"Haematoxylin Eosin (H&E) staining".protocolsonline.com.11 April 2010.Retrieved22 April2018.
- ^Hitokoto H, Morozumi S, Wauke T, Sakai S, Kurata H (1980)."Inhibitory effects of spices on growth and toxin production of toxigenic fungi".Appl. Environ. Microbiol.39(4): 818–22.doi:10.1128/AEM.39.4.818-822.1980.PMC291425.PMID6769391.
- ^ab"Van Gogh's Fading Colors Inspire Scientific Inquiry".
