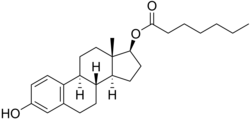
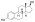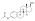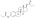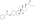Estradiol enantate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Perlutal, Topasel, Unalmes, Yectames, others |
| Other names | EEn; E2-EN; EE; E2E; Estradiol enanthate; Estradiol heptanoate; SQ-16150 |
| Routes of administration | Intramuscular injection[1][2] |
| Drug class | Estrogen;Estrogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokineticdata | |
| Bioavailability | IM:High |
| Protein binding | Estradiol: ~98% (toalbuminandSHBG)[3][4] |
| Metabolism | Cleavageviaesterasesin theliver,blood,andtissues[5][6] |
| Metabolites | Estradiol,heptanoic acid,andmetabolitesof estradiol[5][6] |
| Eliminationhalf-life | IM:5.6–7.5 days[7][1][8][9] |
| Duration of action | IM(10 mg): ~20–30 days[10][5] |
| Excretion | Urine[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.023.272 |
| Chemical and physical data | |
| Formula | C25H36O3 |
| Molar mass | 384.560g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Estradiol enantate(EEnorE2-EN), also spelledestradiol enanthateand sold under the brand namesPerlutalandTopaselamong others, is anestrogenmedication which is used inhormonal birth controlfor women.[1][2][11]It is formulated incombinationwithdihydroxyprogesterone acetophenide(DHPA; algestone acetophenide), aprogestin,and is used specifically as acombined injectable contraceptive.[1][2]Estradiol enantate is not available for medical use alone.[12][13][14][15]The medication, in combination with DHPA, is given byinjection into muscleonce a month.[1][2]
Side effectsof estradiol enantate includebreast tenderness,breast enlargement,nausea,headache,andfluid retention.[16]Estradiol enantate is anestrogenand hence is anagonistof theestrogen receptor,thebiological targetofestrogenslikeestradiol.[6][5]It is anestrogen esterand a long-lastingprodrugofestradiolin the body.[5][6]Because of this, it is considered to be anaturalandbioidenticalform of estrogen.[5][17]
Estradiol enantate was first described by 1954,[18]and was first studied in combination with DHPA as a combined injectable contraceptive in 1964.[19][20]The combination was introduced for clinical use by the mid-1970s.[21][22][23]Estradiol enantate is not available as a standalone medication (i.e., by itself without DHPA).[15]The combination is available inLatin AmericaandHong Kong,and was also previously marketed inSpainandPortugal.[15][2][13]
Medical uses
[edit]Estradiol enantate is used in combination with the progestin DHPA as a once-monthly combined injectable contraceptive for women inLatin AmericaandHong Kong.[1][2][24][15]Estradiol enantate has been studied infeminizing hormone therapyfor transgender women as well.[25]The combination of estradiol enantate and DHPA has likewise been used by transgender women for such purposes.[26]
Available forms
[edit]The following forms of estradiol enantate are or have been available for use:[11][27][28][23][2]
- Estradiol enantate 10 mg and DHPA 150 mg (brand names Perlutal, Topasel, many others)
- Estradiol enantate 5 mg and DHPA 75 mg (brand names Anafertin, Patector NF, Yectames)
- Estradiol enantate 10 mg and DHPA 120 mg (brand names Unalmes, Yectuna)
- Estradiol enantate 10 mg and DHPA 75 mg (brand name Ova Repos; discontinued)
A 6 mg estradiol enantate and 90 mg DHPA formulation was also studied, but was never marketed.[29][30][31]The combination of estradiol enantate and DHPA has also been studied at other doses ranging from 5 to 50 mg estradiol enantate and 75 to 200 mg DHPA.[32]
The combination of estradiol enantate and DHPA is provided inampoulesat estradiol enantate concentrations of 5 mg/mL and 10 mg/mL.
Contraindications
[edit]Contraindicationsof estrogens includecoagulationproblems,cardiovascular diseases,liver disease,and certainhormone-sensitive cancerssuch asbreast cancerandendometrial cancer,among others.[33][34][35][36]
Side effects
[edit]Theside effectsof estradiol enantate are the same as those of estradiol. Examples of such side effects includebreast tendernessandenlargement,nausea,bloating,edema,headache,andmelasma.[16]The combination of estradiol enantate and DHPA as a combined injectable contraceptive has shown no adverse effects onliverfunction,lipid metabolism,orcoagulation.[37][2]
ABraziliancase reportof aprolactinomain atransgender womantreated with 10 mg estradiol enantate every 2 weeks exists.[38][39]WhileDHPAwas not mentioned in this instance,[38][39]estradiol enantate is normally formulated in combination with DHPA including in Brazil.[12][14]
Overdose
[edit]Symptomsof estrogenoverdosagemay includenausea,vomiting,bloating,increased weight,water retention,breast tenderness,vaginal discharge,heavy legs,andleg cramps.[33]These side effects can be diminished by reducing the estrogen dosage.[33]
Interactions
[edit]Inhibitorsandinducersofcytochrome P450may influence themetabolismof estradiol and by extension circulating estradiol levels.[40]
Pharmacology
[edit]
Pharmacodynamics
[edit]Estradiol enantate is anestradiol ester,or aprodrugofestradiol.[5][6]As such, it is anestrogen,or anagonistof theestrogen receptors.[5][6]Estradiol enantate is of about 41% highermolecular weightthan estradiol due to the presence of its C17βenantateester.[41][15]Because estradiol enantate is a prodrug of estradiol, it is considered to be anaturalandbioidenticalform of estrogen.[5][17]
The combination of 10 mg estradiol enantate and 150 mg DHPA as a once-monthly combined injectable contraceptive (which achieves levels of estradiol of around 350 pg/mL)[10][42][43]has been found to have little to no effect on many markers of estrogen-modulatedliver protein synthesis,including circulating levels ofHDLandLDL cholesterol,copper,ceruloplasmin,total and freecortisol,corticosteroid-binding globulin,andsex hormone-binding globulin.[44][45]However, it was found to significantly increase levels oftriglyceridesand to significantly decrease levels of total and freetestosterone.[45]In contrast to the estradiol enantate-containing combined injectable contraceptive, low-doseethinylestradiol-containingbirth control pillsproduce highly significant changes in all of the preceding parameters.[44][45]
Studies in women and femalecapuchin monkeyshave found that injections of estradiol enantate and DHPA significantly alter levels ofcoagulation factors.[46][47]
The clinicalestrogeniceffects of estradiol enantate andethinylestradiolhave been compared in other studies as well.[48]
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
| Estradiol enanthate | Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources
Notes:Allaqueous suspensionsare ofmicrocrystallineparticle size.Estradiolproduction during themenstrual cycleis 30–640 µg/d (6.4–8.6 mg total per month or cycle). Thevaginalepitheliummaturation dosage ofestradiol benzoateorestradiol valeratehas been reported as 5 to 7 mg/week. An effectiveovulation-inhibiting doseofestradiol undecylateis 20–30 mg/month.Sources:See template. | |||||
Pharmacokinetics
[edit]When estradiol enantate is administered in anoil solutionbyintramuscular injection,adepoteffect occurs, and this results in it having a longduration of action.[10][6][49]The duration of action of estradiol enantate is considerably longer than that of various otherestradiol esters,such asestradiol benzoateandestradiol valerate,whereas its duration is shorter than that ofestradiol undecylate.[10][50][51]In general, the longer thefatty acidesterchain,the morelipophilicthe estradiol ester, the more slowly it is released from the depot and absorbed into the circulation, and the longer its duration of action.[6][49]
Thepharmacokineticsof estradiol enantate have been assessed in a number of studies.[10][52][42][7][43][53]It has usually been studied in combination with DHPA.[10][52][42][43]Following anintramuscular injectionof estradiol enantate, levels of estradiol have been found to peak after 3 to 8 days.[10][43][7]Maximal levels of estradiol after a 5 mg injection of estradiol enantate have been found to be about 163 to 209 pg/mL and after a 10 mg injection of estradiol enantate have been found to be about 283 to 445 pg/mL.[10][42][43]However, one outlying study reported peak estradiol levels of 850 pg/mL after an intramuscular injection of 10 mg estradiol enantate in three postmenopausal women.[7]It usedradioimmunoassayfor the determinations, with no mention ofchromatographic separation.[7]Estradiol levels following an intramuscular injection of 10 mg estradiol enantate have been found to return to baseline levels of around 50 pg/mL after about 20 to 30 days.[42][7][5][53][10]However, ametabolicstudy found that traces ofradiolabeledestradiol enantate remained detectable in blood for at least 30 to 40 days and for as long as 60 days.[52]Studies have reported that theelimination half-lifeof estradiol enantate after a single 10 mg intramuscular injection was 5.6 to 7.5 days.[7][1][8]Thevolume of distributionof estradiol enantate has been reported to be 5.087 L.[9]Estradiol enantate isexcretedpreferentially inurine.[22]
There were concerns about possible accumulation of estradiol enantate and consequent estrogenic overexposure with once-monthly combined injectable contraceptives containing the medication due its longduration,and this may have limited the use of such combined injectable contraceptives.[8][10]Subsequent clinical studies have found that there is very limited or no accumulation of estradiol enantate when it is used in once-a-month injectable contraceptives.[8][37][2]
-
Estradiol levels after the most recent intramuscular injection during once-monthly 5 or 10 mg estradiol enanthate and 75 or 150 mgdihydroxyprogesterone acetophenidecontraception in one premenopausal woman each.[42]Assays were performed usingradioimmunoassay.[42]Source was Recio et al. (1986).[42]
-
Estradiol levels after a single intramuscular injection of 10 mg estradiol enanthate in three postmenopausal women.[7]Assays were performed usingradioimmunoassay.[7]Source was Wiemeyer et al. (1986).[7]
-
Estradiol andprolactinlevels after the most recent intramuscular injection during once-monthly 10 mg estradiol enanthate and 150 mgdihydroxyprogesterone acetophenidecontraception in 10 premenopausal women.[53]Only four determinations were made: days 0, 10, 20, and 30.[53]Assays were performed usingradioimmunoassay.[53]Source was Garza-Flores et al. (1989).[53]
Chemistry
[edit]Estradiol enantate, also known as estradiol 17β-enantate or estra-1,3,5(10)-triene-3,17β-diol 17β-heptanoate, is asyntheticestranesteroidand the C17βenantate(heptanoate)fatty acidesterofestradiol.[41][15]Other common esters of estradiol used clinically includeestradiol benzoate,estradiol cypionate,estradiol undecylate,andestradiol valerate.[15]Estradiol dienantate(component ofClimacteron), or estradiol 3,17β-dienantate, has also been used.[41][54][55][56]
The experimentaloctanol/water partition coefficient(logP) of estradiol enanthate is 6.7.[57]
| Estrogen | Structure | Ester(s) | Relative mol. weight |
Relative E2contentb |
log Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzoic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid(×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid,butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Cyclic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid(×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid(×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine,phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes:a= Length ofesterincarbonatomsforstraight-chain fatty acidsor approximate length of ester in carbon atoms foraromaticorcyclicfatty acids.b= Relative estradiol content by weight (i.e., relativeestrogenicexposure).c= Experimental or predictedoctanol/water partition coefficient(i.e.,lipophilicity/hydrophobicity). Retrieved fromPubChem,ChemSpider,andDrugBank.d= Also known asestradiol normustine phosphate.e=Polymerofestradiol phosphate(~13repeat units).f= Relative molecular weight or estradiol content per repeat unit.g= log P of repeat unit (i.e., estradiol phosphate).Sources:See individual articles. | |||||||||
History
[edit]Estradiol enantate was first described, along with a variety of otherestradiol esters,by Karl Junkmann ofSchering AGin 1953.[58][18][59][60][51][61][62]The first clinical study of estradiol enantate and DHPA as acombined injectable contraceptivewas conducted in 1964.[19][20]The combination was marketed by the mid-1970s.[21][22][23]
Society and culture
[edit]Generic names
[edit]Estradiol enantateis theBritish Englishgeneric nameof the medication and itsINNMandBANM,whileestradiol enanthateis itsUSANandAmerican Englishgeneric name.[41][15][12][63]Its generic names in other languages are as follows:[13][12]
- French:enantate d'estradiolandestradiol enantate
- German:estradiol enantat
- Italian:estradiolo enantato
- PortugueseandSpanish:enantato de estradiolandestradiol enantato
Estradiol enantate is also known by its former developmental code nameSQ-16150.[64]It has been referred to asestradiol heptanoate.[15][41][14][12][13]
Brand names
[edit]Estradiol enantate has been marketed under a wide variety of brand names.[13][12][65][66][11][67][28][68][23][2][10]It has been marketed in a few different preparations, with varying doses of estradiol enantate and DHPA.[28][11][67][27][23][2][10]These formulations all have different brand names, which include the following (†= discontinued):[13][12][65][66][27][28][11][67][2][69]
- EEn 10 mg / DHPA 150 mg: Acefil, Agurin†,Atrimon†,Ciclomes, Ciclovar, Ciclovular, Cicnor†,Clinomin, Cycloven, Daiva, Damix, Deprans, Deproxone, Exuna, Ginestest, Ginoplan†,Gynomes, Horprotal, Listen, Luvonal, Neogestar, Neolutin, Nomagest, Nonestrol, Normagest, Normensil, Novular, Oterol, Ovoginal, Patector, Patectro, Perludil, Perlumes, Perlutal, Perlutale, Perlutan, Perlutin, Perlutin-Unifarma, Permisil, Preg-Less, Pregnolan, Progestrol†,Protegin, Proter, Seguralmes, Synovular, Topasel, Unigalen, Uno-Ciclo, and Vagital.
- EEn 10 mg / DHPA 120 mg: Anafertin†,Patector NF, and Yectames.
- EEn 5 mg / DHPA 75 mg: Unalmes and Yectuna.
- EEn 10 mg / DHPA 75 mg: Ova Repos†.
- Unsorted: Evitas†,Femineo†,and Primyfar†.
The combination of EEn 10 mg and DHPA 150 mg was developed under the developmental brand name Deladroxate, but this brand name was never used commercially.[23][2]
Availability
[edit]
Estradiol enantate has been marketed in combination with DHPA as acombined injectable contraceptivein at least 19 countries, mostly inLatin America.[11][67][28][68][13][12][65][66]A few different preparations, with varying doses of EEn and DHPA and varying availability, have been introduced.[28][11][67][27][23][2][10]These formulations have the following approval and availability (†= discontinued in this country):[13][12][65][66][27][28][11][67][2]
- EEn 10 mg / DHPA 150 mg: at least 19 countries, includingArgentina,Belize,Brazil,Chile,Colombia,Costa Rica,theDominican Republic,Ecuador,El Salvador,Guatemala,Honduras,Hong Kong,Mexico,Nicaragua,Panama,Paraguay,Peru,Portugal†,andSpain†.
- EEn 10 mg / DHPA 120 mg: at least 3 countries, includingBrazil†,Chile,andParaguay.
- EEn 5 mg / DHPA 75 mg: at least 9 countries, includingCosta Rica,theDominican Republic,El Salvador,Guatemala,Honduras,Mexico,Nicaragua,Panama,andSpain†.
EEn is also available inCanadain combination withestradiol benzoateandtestosterone enantateforveterinaryuse as Uni-Bol.[70]
Usage
[edit]EEn/DHPA is the most widely used combined injectable contraceptive in Latin America.[71]It was estimated in 1995 that EEn/DHPA was used as a combined injectable contraceptive in Latin America by at least 1 million women.[28]However, combined injectable contraceptives like EEn/DHPA are unlikely to constitute a large proportion of total contraceptive use in the countries in which they are available.[28]
See also
[edit]References
[edit]- ^abcdefghJarquín González JD, Elda de Aguirre L, Rodríguez C, Abrego de Aguilar M, Carrillo F, León DA, et al. (September 1996). "Dihydroxyprogesterone acetophenide 150 mg + estradiol enantate 10 mg as monthly injectable contraceptives".Advances in Contraception.12(3): 213–225.doi:10.1007/BF01849664.PMID8910663.S2CID2522426.
- ^abcdefghijklmnoNewton JR, D'arcangues C, Hall PE (1994). "A review of" once-a-month "combined injectable contraceptives".Journal of Obstetrics and Gynaecology.4(Suppl 1): S1-34.doi:10.3109/01443619409027641.PMID12290848.
- ^Stanczyk FZ, Archer DF, Bhavnani BR (June 2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment".Contraception.87(6): 706–727.doi:10.1016/j.contraception.2012.12.011.PMID23375353.
- ^Falcone T, Hurd WW (2007).Clinical Reproductive Medicine and Surgery.Elsevier Health Sciences. pp. 22, 362, 388.ISBN978-0-323-03309-1.
- ^abcdefghijOettel M, Schillinger E (6 December 2012).Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen.Springer Science & Business Media. pp. 261, 271.ISBN978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens. [...] Wiemeyer et al. (1986) measured elevated estradiol levels up to 31 days after an intramuscular dose of 10mg estradiol enanthate.
- ^abcdefghKuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration".Climacteric.8(Suppl 1): 3–63.doi:10.1080/13697130500148875.PMID16112947.S2CID24616324.
- ^abcdefghijWiemeyer JC, Fernandez M, Moguilevsky JA, Sagasta CL (November 1986). "Pharmacokinetic studies of estradiol enantate in menopausic women".Arzneimittel-Forschung.36(11): 1674–1677.PMID3814225.
- ^abcdSang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives".Contraception.49(4): 361–385.doi:10.1016/0010-7824(94)90033-7.PMID8013220.
- ^ab"Bula do Algestona Acetofenida + Enantato de Estradiol".Consulta Remédios.Archived fromthe originalon 18 September 2018.Retrieved18 September2018.
- ^abcdefghijklmnopqGarza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives".Contraception.49(4): 347–359.doi:10.1016/0010-7824(94)90032-9.PMID8013219.
- ^abcdefghBagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014)."Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control"(PDF).World J Pharm Pharm Sci.3(10): 364–392.ISSN2278-4357.
- ^abcdefghiSweetman SC, ed. (2009). "Sex hormones and their modulators".Martindale: The Complete Drug Reference(36th ed.). London: Pharmaceutical Press. p. 2082.ISBN978-0-85369-840-1.
- ^abcdefgh"Micromedex Products: Please Login".
- ^abc"Estradiol: Uses, Dosage & Side Effects".
- ^abcdefghiIndex Nominum 2000: International Drug Directory.Taylor & Francis US. 2000. p. 405.ISBN978-3-88763-075-1.Retrieved20 May2012.
- ^abGhosh AK (23 September 2010).Mayo Clinic Internal Medicine Board Review.OUP USA. pp. 222–.ISBN978-0-19-975569-1.
- ^abArun N, Narendra M, Shikha S (15 December 2012).Progress in Obstetrics and Gynecology--3.Jaypee Brothers Medical Publishers Pvt. Ltd. pp. 419–.ISBN978-93-5090-575-3.
- ^abInternational Neurochemical Symposium Proceedings.Academic Press. 1954. p. 453.
- ^abRutherford RN, Banks AL, Coburn WA (1964)."Deladroxate for the Prevention of Ovulation".Fertility and Sterility.15(6): 648–652.doi:10.1016/s0015-0282(16)35410-3.PMID14236841.
- ^abTaymor ML, Planck S, Yahia C (1964)."Ovulation Inhibition with a Long-Acting Parenteral Progestogen-Estrogen Combination".Fertility and Sterility.15(6): 653–660.doi:10.1016/s0015-0282(16)35411-5.PMID14236842.
- ^abBringer J, Hedon B (15 September 1995).Fertility and Sterility: A Current Overview.CRC Press. pp. 47–.ISBN978-1-85070-694-6.
- ^abcToppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations".Obstetrical & Gynecological Survey.32(6): 335–347.doi:10.1097/00006254-197706000-00001.PMID865726.
- ^abcdefgToppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives".Contraception.49(4): 293–301.doi:10.1016/0010-7824(94)90029-9.PMID8013216.
- ^Zutshi V, Rathore AM, Sharma K (1 January 2005).Hormones in Obstetrics and Gynaecology.New Delhi: Jaypee Brothers Publishers. p. 138.ISBN978-81-8061-427-9.Retrieved20 May2012.[permanent dead link]
- ^Becerra Fernández A, de Luis Román DA, Piédrola Maroto G (October 1999)."[Morbidity in transsexual patients with cross-gender hormone self-treatment]"[Morbidity in transsexual patients with cross-gender hormone self-treatment](PDF).Medicina Clinica(in Spanish).113(13): 484–487.PMID10604171.
- ^Kulick D (12 January 2009).Travesti: Sex, Gender, and Culture among Brazilian Transgendered Prostitutes.University of Chicago Press. pp. 64–66.ISBN978-0-226-46101-4.
- ^abcdeIARC Working Group on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, International Agency for Research on Cancer (2007).Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy.World Health Organization. pp. 431–433, 467.ISBN978-92-832-1291-1.
- ^abcdefghiIARC Working Group on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer (1 January 1999).Hormonal Contraception and Post-menopausal Hormonal Therapy(PDF).IARC. p. 65.ISBN978-92-832-1272-0.Archived fromthe original(PDF)on 28 August 2021.Retrieved19 September2018.
- ^d'Arcangues C, Snow RC (1999). "Injectable Contraceptives.". In Rabe T, Runnebaum B (eds.).Fertility Control — Update and Trends.pp. 121–149.doi:10.1007/978-3-642-86696-8_6.ISBN978-3-642-86698-2.
- ^Coutinho EM, Spinola P, Barbosa I, Gatto M, Tomaz G, Morais K, et al. (March 1997)."Multicenter, double-blind, comparative clinical study on the efficacy and acceptability of a monthly injectable contraceptive combination of 150 mg dihydroxyprogesterone acetophenide and 10 mg estradiol enanthate compared to a monthly injectable contraceptive combination of 90 mg dihydroxyprogesterone acetophenide and 6 mg estradiol enanthate".Contraception.55(3): 175–181.doi:10.1016/S0010-7824(97)00018-8.PMID9115007.
- ^Coutinho EM, Spinola P, Tomaz G, Morais K, Nassar de Souza R, Sabino Pinho Neto J, et al. (April 2000)."Efficacy, acceptability, and clinical effects of a low-dose injectable contraceptive combination of dihydroxyprogesterone acetophenide and estradiol enanthate".Contraception.61(4): 277–280.doi:10.1016/S0010-7824(00)00099-8.PMID10899484.
- ^Koetsawang S (April 1994). "Once-a-month injectable contraceptives: efficacy and reasons for discontinuation".Contraception.49(4): 387–398.doi:10.1016/0010-7824(94)90034-5.PMID8013221.
- ^abcLauritzen C (September 1990). "Clinical use of oestrogens and progestogens".Maturitas.12(3): 199–214.doi:10.1016/0378-5122(90)90004-P.PMID2215269.
- ^Lauritzen C, Studd JW (22 June 2005).Current Management of the Menopause.CRC Press. pp. 95–98, 488.ISBN978-0-203-48612-2.
- ^Laurtizen C (2001). "Hormone Substitution Before, During and After Menopause". In Fisch FH (ed.).Menopause – Andropause: Hormone Replacement Therapy Through the Ages(PDF).Krause & Pachernegg: Gablitz. pp. 67–88.ISBN978-3-901299-34-6.
- ^Midwinter A (1976). "Contraindications to estrogen therapy and management of the menopausal syndrome in these cases". In Campbell S (ed.).The Management of the Menopause & Post-Menopausal Years: The Proceedings of the International Symposium held in London 24–26 November 1975 Arranged by the Institute of Obstetrics and Gynaecology, The University of London.MTP Press Limited. pp. 377–382.doi:10.1007/978-94-011-6165-7_33.ISBN978-94-011-6167-1.
- ^abDe Aguilar MA, Altamirano L, Leon DA, De Fung RC, Grillo AE, Gonzalez JD, et al. (December 1997). "Current status of injectable hormonal contraception, with special reference to the monthly method".Advances in Contraception.13(4): 405–417.doi:10.1023/A:1006501526018.PMID9404550.S2CID19603384.
- ^abCamara VL, Zanardi UV, Glezer A, Paraiba DB, Bronstein MD, Mendonca BB, Costa EM (June 2010)."Estrogen as a Presumed Risk Factor for Prolactinoma in a Male-to-Female Transsexual Patient"(PDF).Endocrine Reviews.31(3, Supplement 1): S347.
- ^abCamara VL (July 2010). "Estradiol enantate First report of prolactinoma, in a transsexual".Reactions.24(1311): 24.doi:10.2165/00128415-201013110-00077.S2CID195175382.
- ^Cheng ZN, Shu Y, Liu ZQ, Wang LS, Ou-Yang DS, Zhou HH (February 2001). "Role of cytochrome P450 in estradiol metabolism in vitro".Acta Pharmacologica Sinica.22(2): 148–154.PMID11741520.
- ^abcdeElks J (14 November 2014).The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies.Springer. pp. 898–.ISBN978-1-4757-2085-3.
- ^abcdefghRecio R, Garza-Flores J, Schiavon R, Reyes A, Diaz-Sanchez V, Valles V, et al. (June 1986). "Pharmacodynamic assessment of dihydroxyprogesterone acetophenide plus estradiol enanthate as a monthly injectable contraceptive".Contraception.33(6): 579–589.doi:10.1016/0010-7824(86)90046-6.PMID3769482.
- ^abcdefSchiavon R, Benavides S, Oropeza G, Garza-Flores J, Recio R, Díaz-Sanchez V, Pérez-Palacios G (June 1988). "Serum estrogens and ovulation return in chronic users of a once-a-month injectable contraceptive".Contraception.37(6): 591–598.doi:10.1016/0010-7824(88)90005-4.PMID3396358.
- ^abWiemeyer JC, Vidal M, Gallardo, E (March 1995). "IX International Congress. Session 22 Long-Acting Contraception II. Abstracts. Experiences with dihydroxyprogesterone acetophenide (DHPA) 150 mg plus estradiol enanthate (E2EN) 10 mg as a once a month injectable contraceptive in Latin America".Advances in Contraception.11:54–60.doi:10.1007/BF02436103.S2CID75854488.
- ^abcWiemeyer JC, Sagasta CL, Roncales Mateo JM, Lavarello AC, Angel de Toro LA, Salas Diaz R (July 1990). "Multicentred clinical study of the metabolic effect of the monthly injectable contraceptive containing dihydroxyprogesterone acetophenide 150 mg + estradiol enanthate 10 mg".Contraception.42(1): 13–28.doi:10.1016/0010-7824(90)90088-D.PMID2117515.
- ^Oliva Filho, W. M., & Santos, N. da C. (1992). Efeitos na coagulação sanguinea em usuárias da associação acetofenido de dihidroxiprogesterona 150mg e enantato de estradiol 10mg como metodo anticoncepcional injetavel. Universidade de São Paulo, São Paulo.https://bdpi.usp.br/item/000736190
- ^Duarte RC, Belham FS, Tavares MC (2018)."Risco de doenca tromboliticas apos o uso de algestona acetofenida e enantato de estradiol"[Risk of thrombolytic disease after the use of algestone acetophenide and estradiol enanthate].Revista de Patologia do Tocantins[Journal of Pathology of Tocantins] (in Portuguese).5(1): 17.doi:10.20873/uft.2446-6492.2018v5n1p17.ISSN2446-6492.
- ^Moguilevsky JA, Wiemeyer JC, Sagasta CL, Leiderman S (November 1986). "Estrogenic activities of estradiol enantate and ethinylestradiol compared at a clinical level".Arzneimittel-Forschung.36(11): 1671–1674.PMID3101711.
- ^abVermeulen A (1975). "Longacting steroid preparations".Acta Clinica Belgica.30(1): 48–55.doi:10.1080/17843286.1975.11716973.PMID1231448.
- ^Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (April 1980). "A comparison of the pharmacokinetic properties of three estradiol esters".Contraception.21(4): 415–424.doi:10.1016/s0010-7824(80)80018-7.PMID7389356.
- ^abWilde PR, Coombs CF, Short AJ (1959).The Medical Annual: A Year Book of Treatment and Practitioner's Index...Publishing Science Group.
As in the case of progestogens the esters of oestradiol vary in the duration of their effect. Oestradiol benzoate is short-acting (three days to a week). Oestradiol valerianate is somewhat longer-acting, and oestradiol enanthate and undecylate have considerably more prolonged duration of effectiveness. The undecylate may remain effective for some months, and should not be employed, [...]
- ^abcGual C, Pérez-Palacios G, Perez AE, Ruiz MR, Solis J, Cervantes A, et al. (1973). "Metabolic fate of a long-acting injectable estrogen-progestogen contraceptive 1,2".Contraception.7(4): 271–287.doi:10.1016/0010-7824(73)90145-5.ISSN0010-7824.
- ^abcdefGarza-Flores J, Alba VM, Cravioto MC, Hernandez L, Perez-Palacios G, Alvarado G, et al. (May 1989). "Estrogen-progestogen once-a-month injectable contraceptives and serum prolactin".Contraception.39(5): 519–529.doi:10.1016/0010-7824(89)90107-8.PMID2524362.
- ^Ginsburg ES (1999). "Androgen Replacement in Postmenopausal Women". In Seifer DB, Kennard EA (eds.).Menopause: Endocrinology and Management.Vol. 18. pp. 209–219.doi:10.1007/978-1-59259-246-3_13.ISBN978-1-61737-129-5.
- ^Greenblatt RB, Barfield WE, Jungck EC (January 1962)."The treatment of the menopause".Canadian Medical Association Journal.86(3): 113–114.PMC1848811.PMID13901504.
- ^Harlow BL, Abraham ME (27 July 1999)."Depression in Menopause".In Seifer DB, Kennard EA (eds.).Menopause: Endocrinology and Management.Springer Science & Business Media. pp. 183–.doi:10.1007/978-1-59259-246-3_7.ISBN978-1-59259-246-3.
- ^"Estradiol enanthate | C25H36O3".ChemSpider.
- ^Junkmann K (1953). "Über protrahiert wirksame Östrogene" [Over protracted effective estrogens].Naunyn-Schmiedebergs Archiv für Experimentelle Pathologie und Pharmakologie.220(5).doi:10.1007/BF00246561.ISSN0028-1298.S2CID20753905.
- ^Waelsch H (1955).Biochemistry of the Developing Nervous System: Proceedings.Academic Press. p. 453.
- ^Acta Cytologica.International Academy of Cytology. 1958. p. 378.
- ^Gauthier B, Le Dreff L, Aubry R (1958). "Hormone derivatives of long-lasting action. I. Esters of estradiol".Annales Pharmaceutiques Françaises.16:757–66.ISSN0003-4509.
Treating 10 g. estradiol benzoate in 30 cc.dry C5H5N dropwise with 4.3 g. n-C6H13COCl (b20 71-2°), heating 1 hr. at 50-60°, pouring into 100 cc. 10% H2SO4, sepg. the oil after its solidification, washing with petr. ether, heating with 50 cc. MeOH, and cooling gave 10 g. 17-heptoyl-3β-benzoylestradiol, m. 95-8°. Dissolving 10 g. of this in 210 cc. 0.1N NaOH in MeOH and 40 cc. Me2CO with stirring, adding HCl to pH 7, filtering, evapg. in vacuo, and stirring the residue with petr. ether gave 7.9 g. 17-heptoyl-β-estradiol, m. 94-6° (iso-Pr2O). Adding to 5 g. estradiol stirred in 10 cc. anhyd. pyridine 8 g. n-C10H21COCl (b20 135-6°), keeping 1 hr. at 100°, cooling, adding 50 cc. 10% H2SO4, dissolving the sepd. ester in 50 cc. iso-Pr2O, washing with satd. NaHCO3 soln. and H2O, drying, and evapg. at room temp. gave 10.7 g. 3,17-diundecanoylestradiol, m. 48-9° (MeOH-Me2CO, then Me2O-Et2O), λmax. (0.005% in MeOH contg. 4% iso-Pr2O) 268 mμ, λmin. 282 and 250 mμ, inflexion 215 mμ. Stirring 8.8 g. estradiol divalerate in 90 cc. MeOH and 0.4 g. NaOH under N 210 min. to soln., adding 20% HCl to pH 7, evapg. in vacuo to 10 cc., keeping overnight at a low temp., and washing with H2O, MeOH, and petr. ether gave 4.4 g. 17-valeryl-β-estradiol, m. 145-6°, λmax. (0.005% in EtOH) 282 mμ, λmin. 248 mμ, inflexion 215 mμ. A single dose of 25 mg. of the diundecanate gave a therapeutic effect lasting 3 weeks.
- ^ES 241206A1,Alter SA, "Esters of cortical hormones, androgens, or esterogens by transesterification and alcoholysis", published 1958-07-16
- ^Morton IK, Hall JM (6 December 2012).Concise Dictionary of Pharmacological Agents: Properties and Synonyms.Springer Science & Business Media. pp. 206–.ISBN978-94-011-4439-1.
- ^Milne GW (8 May 2018).Drugs: Synonyms and Properties: Synonyms and Properties.Taylor & Francis. pp. 1404–.ISBN978-1-351-78989-9.
- ^abcd"Archived copy".Archived fromthe originalon 18 September 2018.Retrieved19 September2018.
{{cite web}}:CS1 maint: archived copy as title (link) - ^abcd"Progestin Oral, Parenteral, Vaginal Advanced Patient Information".
- ^abcdefSenanayake P, Potts M (14 April 2008).Atlas of Contraception, Second Edition.CRC Press. pp. 50–.ISBN978-0-203-34732-4.
- ^abLähteenmäki P (6 December 2012)."Intrauterine Hormone-Releasing Systems".In Rabe T, Runnebaum B (eds.).Fertility Control — Update and Trends: Update and Trends.Springer Science & Business Media. pp. 173-184 (183).ISBN978-3-642-86696-8.
Two additional monthly, combined injectable methods warrant mention. Deladroxate (commercially labelled as Perlutan, Topasel, Agurin, Horprotal and Uno-Ciclo in various countries), is a combination of 150 mg dihydroxyprogesterone acetophenide and 10 mg estradiol enanthate, and is available in many Latin American countries and Spain. The method is highly effective, without a single pregnancy reported in large clinical trials (Koetsawang 1994). Although available since the 1960s, the method has not been studied as extensively as Cyclofem or Mesigyna. The original manufacturer withdrew support due to toxicological concerns with dihydroxyprogesterone acetophenide, and clinical evaluations continue to be published. A recent dose-finding trial compared the standard available dose of 150/10 with a lower dose of 90/6, and concluded the lower dose was equally effective (Coutinho et al., 1997).
- ^Gallo MF, Grimes DA, Lopez LM, Schulz KF, d'Arcangues C (2013)."Combination injectable contraceptives for contraception".The Cochrane Database of Systematic Reviews.3:CD004568.doi:10.1002/14651858.CD004568.pub3.PMC6513542.PMID23641480.
- ^"Drug Product Database Online Query".25 April 2012.
- ^Speroff L, Fritz MA (2005).Clinical Gynecologic Endocrinology and Infertility.Lippincott Williams & Wilkins. pp. 969–.ISBN978-0-7817-4795-0.

![Estradiol levels after the most recent intramuscular injection during once-monthly 5 or 10 mg estradiol enanthate and 75 or 150 mg dihydroxyprogesterone acetophenide contraception in one premenopausal woman each.[42] Assays were performed using radioimmunoassay.[42] Source was Recio et al. (1986).[42]](https://upload.wikimedia.org/wikipedia/commons/thumb/1/19/Estradiol_levels_after_an_intramuscular_injection_of_different_doses_of_estradiol_enanthate_in_premenopausal_women.png/288px-Estradiol_levels_after_an_intramuscular_injection_of_different_doses_of_estradiol_enanthate_in_premenopausal_women.png)
![Estradiol levels after a single intramuscular injection of 10 mg estradiol enanthate in three postmenopausal women.[7] Assays were performed using radioimmunoassay.[7] Source was Wiemeyer et al. (1986).[7]](https://upload.wikimedia.org/wikipedia/commons/thumb/c/c7/Estradiol_levels_after_a_single_intramuscular_injection_of_10_mg_estradiol_enantate_in_postmenopausal_women.png/292px-Estradiol_levels_after_a_single_intramuscular_injection_of_10_mg_estradiol_enantate_in_postmenopausal_women.png)
![Estradiol and prolactin levels after the most recent intramuscular injection during once-monthly 10 mg estradiol enanthate and 150 mg dihydroxyprogesterone acetophenide contraception in 10 premenopausal women.[53] Only four determinations were made: days 0, 10, 20, and 30.[53] Assays were performed using radioimmunoassay.[53] Source was Garza-Flores et al. (1989).[53]](https://upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Estradiol_and_prolactin_levels_after_the_last_injection_during_therapy_with_estradiol_enantate_and_dihydroxyprogesterone_acetophenide_in_women.png/300px-Estradiol_and_prolactin_levels_after_the_last_injection_during_therapy_with_estradiol_enantate_and_dihydroxyprogesterone_acetophenide_in_women.png)
![Simplified curves of estradiol levels after an intramuscular injection of 10 mg estradiol enanthate (E2-EN) and 150 mg dihydroxyprogesterone acetophenide (DHPA) in oil solution with single or continuous once-monthly use in women.[43][10] Source was Garza-Flores (1994).[10]](https://upload.wikimedia.org/wikipedia/commons/thumb/4/49/Estradiol_levels_with_estradiol_enanthate_and_dihydroxyprogesterone_acetophenide_after_single_or_repeated_injections_in_premenopausal_women.png/280px-Estradiol_levels_with_estradiol_enanthate_and_dihydroxyprogesterone_acetophenide_after_single_or_repeated_injections_in_premenopausal_women.png)
![Simplified curves of estradiol levels after injection of different estradiol esters in women.[10] Source was Garza-Flores (1994).[10]](https://upload.wikimedia.org/wikipedia/commons/thumb/8/8c/Idealized_curves_of_estradiol_levels_after_injection_of_different_estradiol_esters_in_women.png/258px-Idealized_curves_of_estradiol_levels_after_injection_of_different_estradiol_esters_in_women.png)