Krill
| Krill | |
|---|---|

| |
| Northern krill(Meganyctiphanes norvegica) | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Malacostraca |
| Superorder: | Eucarida |
| Order: | Euphausiacea Dana,1852 |
| Families and genera | |
| |
Krill(Euphausiids)[1](sg.:krill) are small and exclusively marinecrustaceansof theorderEuphausiacea,found in all the world's oceans.[2]The name "krill" comes from theNorwegianwordkrill,meaning "smallfryof fish ",[3]which is also often attributed to species of fish.
Krill are considered an importanttrophic levelconnection near the bottom of thefood chain.They feed onphytoplanktonand, to a lesser extent,zooplankton,and are also the main source of food for many larger animals. In theSouthern Ocean,one species, theAntarctic krill,makes up an estimatedbiomassof around 379 milliontonnes,[4]making it among the species with the largest total biomass. Over half of this biomass is eaten by whales,seals,penguins, seabirds,squid,and fish each year. Most krill species display largedaily vertical migrations,providing food for predators near the surface at night and in deeper waters during the day.
Krill are fished commercially in the Southern Ocean and in the waters around Japan. The total global harvest amounts to 150,000–200,000 tonnes annually, mostly from theScotia Sea.Most krill catch is used foraquacultureandaquariumfeeds, asbaitinsport fishing,or in the pharmaceutical industry. Krill are also used for human consumption in several countries. They are known asokiami(オキアミ)in Japan and ascamaronesin Spain and the Philippines. In the Philippines, they are also calledalamangand are used to make a salty paste calledbagoong.
Krill are also the main prey ofbaleen whales,including theblue whale.
Taxonomy
[edit]Krill belong to the largearthropodsubphylum,theCrustacea.The most familiar and largest group of crustaceans, theclassMalacostraca,includes thesuperorderEucaridacomprising the three orders, Euphausiacea (krill),Decapoda(shrimp, prawns, lobsters, crabs), and the planktonicAmphionidacea.
The order Euphausiacea comprises twofamilies.The more abundantEuphausiidaecontains 10 differentgenerawith a total of 85 species. Of these, the genusEuphausiais the largest, with 31 species.[5]The lesser-known family, theBentheuphausiidae,has only onespecies,Bentheuphausia amblyops,abathypelagickrill living in deep waters below 1,000 m (3,300 ft). It is considered the most primitive extant krill species.[6]
Well-known species of the Euphausiidae of commercialkrill fisheriesincludeAntarctic krill(Euphausia superba),Pacific krill(E. pacifica) andNorthern krill(Meganyctiphanes norvegica).[7]
Phylogeny
[edit]| Proposed phylogeny of Euphausiacea[8] | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Phylogeny obtained from morphological data, (♠) names coined in,[8](♣) possibly paraphyletic taxon due toNematobrachionin.[8](♦) clades differs from Casanova (1984),[9]wherePseudoeuphausiais sister toNyctiphanes,Euphausiais sister toThysanopodaandNematobrachionis sister toStylocheiron. |
As of 2013[update],the order Euphausiacea is believed to bemonophyleticdue to several unique conserved morphological characteristics (autapomorphy) such as its naked filamentous gills and thin thoracopods[10]and by molecular studies.[11][12][13]
There have been many theories of the location of the order Euphausiacea. Since the first description ofThysanopode tricuspidebyHenri Milne-Edwardsin 1830, the similarity of their biramous thoracopods had led zoologists to group euphausiids and Mysidacea in the orderSchizopoda,which was split byJohan Erik Vesti Boasin 1883 into two separate orders.[14]Later,William Thomas Calman(1904) ranked theMysidaceain the superorderPeracaridaand euphausiids in the superorderEucarida,although even up to the 1930s the order Schizopoda was advocated.[10]It was later also proposed that order Euphausiacea should be grouped with thePenaeidae(family of prawns) in the Decapoda based on developmental similarities, as noted byRobert GurneyandIsabella Gordon.[15][16]The reason for this debate is that krill share some morphological features of decapods and others of mysids.[10]
Molecular studies have not unambiguously grouped them, possibly due to the paucity of key rare species such asBentheuphausia amblyopsin krill andAmphionides reynaudiiin Eucarida. One study supports the monophyly of Eucarida (with basal Mysida),[17]another groups Euphausiacea with Mysida (the Schizopoda),[12]while yet another groups Euphausiacea withHoplocarida.[18]
Timeline
[edit]No extant fossil can be unequivocally assigned to Euphausiacea. Some extincteumalacostracantaxahave been thought to be euphausiaceans such asAnthracophausia,Crangopsis—now assigned to theAeschronectida(Hoplocarida)[8]—andPalaeomysis.[19]All dating ofspeciationevents were estimated bymolecular clockmethods, which placed the last common ancestor of the krill family Euphausiidae (order Euphausiacea minusBentheuphausia amblyops) to have lived in theLower Cretaceousabout130million years ago.[12]
Distribution
[edit]Krill occur worldwide in all oceans, although many individual species haveendemicorneritic(i.e.,coastal) distributions.Bentheuphausia amblyops,abathypelagicspecies, has acosmopolitan distributionwithin its deep-sea habitat.[20]
Species of the genusThysanoessaoccur in bothAtlanticandPacificoceans.[21]The Pacific is home toEuphausia pacifica.Northern krill occur across the Atlantic from theMediterranean Seanorthward.
Species with neritic distributions include the four species of the genusNyctiphanes.[22]They are highly abundant along theupwellingregions of theCalifornia,Humboldt,Benguela,andCanariascurrent systems.[23][24][25]Another species having only neritic distribution isE. crystallorophias,which is endemic to the Antarctic coastline.[26]
Species with endemic distributions includeNyctiphanes capensis,which occurs only in the Benguela current,[22]E. mucronatain the Humboldt current,[27]and the sixEuphausiaspecies native to the Southern Ocean.
In the Antarctic, seven species are known,[28]one in genusThysanoessa(T. macrura) and six inEuphausia.TheAntarctic krill(Euphausia superba) commonly lives at depths reaching 100 m (330 ft),[29]whereas ice krill (Euphausia crystallorophias) reach depth of 4,000 m (13,100 ft), though they commonly inhabit depths of at most 300–600 m (1,000–2,000 ft).[30]Krill perform Diel Vertical Migrations (DVM) in large swarms, and acoustic data has shown these migrations to go up to 400 metres in depth.[31]Both are found atlatitudessouth of55° S,withE. crystallorophiasdominating south of74° S[32]and in regions ofpack ice.Other species known in theSouthern OceanareE. frigida,E. longirostris,E. triacanthaandE. vallentini.[33]
Anatomy and morphology
[edit]

Krill arecrustaceansand, like all crustaceans, they have achitinousexoskeleton.They have anatomy similar to a standarddecapodwith their bodies made up of threeparts:the cephalothorax is composed of theheadand thethorax,which are fused, and theabdomen,which bears the ten swimming appendages, and thetail fan.This outer shell of krill is transparent in most species.
Krill feature intricatecompound eyes.Some species adapt to different lighting conditions through the use of screeningpigments.[34]
They have twoantennaeand several pairs of thoracic legs calledpereiopodsorthoracopods,so named because they are attached to the thorax. Their number varies among genera and species. These thoracic legs include feeding legs and grooming legs.
Krill are probably the sister clade of decapods because all species have five pairs ofswimming legscalled "swimmerets" in common with the latter, very similar to those of alobsterorfreshwater crayfish.
In spite of having ten swimmerets, otherwise known aspleopods,krill cannot be considered decapods. They lack any true ground-based legs due to all theirpereiopodshaving been converted into grooming and auxiliary feeding legs. InDecapoda,there are ten functioningpereiopods,giving them their name; whereas here there are no remaining locomotivepereiopods.Nor are there consistently tenpereiopodsat all.
Most krill are about 1–2 centimetres (0.4–0.8 in) long as adults. A few species grow to sizes on the order of 6–15 centimetres (2.4–5.9 in). The largest krill species,Thysanopoda cornuta,livesdeep in the open ocean.[35]Krill can be easily distinguished from other crustaceans such as trueshrimpby their externally visiblegills.[36]
Except forBentheuphausia amblyops,krill arebioluminescentanimals having organs calledphotophoresthat can emit light. The light is generated by anenzyme-catalysedchemiluminescencereaction, wherein aluciferin(a kind of pigment) is activated by aluciferaseenzyme. Studies indicate that the luciferin of many krill species is afluorescenttetrapyrrolesimilar but not identical todinoflagellateluciferin[37]and that the krill probably do not produce this substance themselves but acquire it as part of their diet, which contains dinoflagellates.[38]Krill photophores are complex organs with lenses and focusing abilities, and can be rotated by muscles.[39]The precise function of these organs is as yet unknown; possibilities include mating, social interaction or orientation and as a form of counter-illumination camouflage to compensate their shadow against overhead ambient light.[40][41]
Ecology
[edit]
Feeding
[edit]Many krill arefilter feeders:[24]their frontmostappendages,the thoracopods, form very fine combs with which they can filter out their food from the water. These filters can be very fine in species (such asEuphausiaspp.) that feed primarily onphytoplankton,in particular ondiatoms,which are unicellularalgae.Krill are mostlyomnivorous,[43]although a few species arecarnivorous,preying on smallzooplanktonand fishlarvae.[44]
Krill are an important element of the aquaticfood chain.Krill convert theprimary productionof their prey into a form suitable for consumption by larger animals that cannot feed directly on the minuscule algae. Northern krill and some other species have a relatively small filtering basket and actively huntcopepodsand larger zooplankton.[44]
Predation
[edit]Many animals feed on krill, ranging from smaller animals like fish or penguins to larger ones likesealsandbaleen whales.[45]
Disturbances of anecosystemresulting in a decline in the krill population can have far-reaching effects. During acoccolithophorebloom in theBering Seain 1998,[46]for instance, the diatom concentration dropped in the affected area. Krill cannot feed on the smaller coccolithophores, and consequently the krill population (mainlyE. pacifica) in that region declined sharply. This in turn affected other species: theshearwaterpopulation dropped. The incident was thought to have been one reasonsalmondid not spawn that season.[47]
Several single-celledendoparasitoidicciliatesof the genusColliniacan infect species of krill and devastate affected populations. Such diseases were reported forThysanoessainermisin the Bering Sea and also forE. pacifica,Thysanoessa spinifera,andT. gregariaoff the North American Pacific coast.[48][49]Someectoparasitesof the familyDajidae(epicarideanisopods) afflict krill (and also shrimp andmysids); one such parasite isOculophryxusbicaulis,which was found on the krillStylocheiron affineandS. longicorne.It attaches itself to the animal's eyestalk and sucks blood from its head; it apparently inhibits the host's reproduction, as none of the afflicted animals reached maturity.[50]
Climate changeposes another threat to krill populations.[51]
Plastics
[edit]Preliminary research indicates krill can digestmicroplasticsunder 5 mm (0.20 in) in diameter, breaking them down and excreting them back into the environment in smaller form.[52]
Life history and behavior
[edit]
The life cycle of krill is relatively well understood, despite minor variations in detail from species to species.[15][24]After krill hatch, they experience several larval stages—nauplius,pseudometanauplius,metanauplius,calyptopsis,andfurcilia,each of which divides into sub-stages. The pseudometanauplius stage is exclusive to species that lay their eggs within an ovigerous sac: so-called "sac-spawners". The larvae grow andmoultrepeatedly as they develop, replacing their rigid exoskeleton when it becomes too small. Smaller animals moult more frequently than larger ones.Yolkreserves within their body nourish the larvae through metanauplius stage.
By the calyptopsis stagesdifferentiationhas progressed far enough for them to develop a mouth and a digestive tract, and they begin to eat phytoplankton. By that time their yolk reserves are exhausted and the larvae must have reached thephotic zone,the upper layers of the ocean where algae flourish. During the furcilia stages, segments with pairs of swimmerets are added, beginning at the frontmost segments. Each new pair becomes functional only at the next moult. The number of segments added during any one of the furcilia stages may vary even within one species depending on environmental conditions.[53]After the final furcilia stage, an immature juvenile emerges in a shape similar to an adult, and subsequently developsgonadsand matures sexually.[54]
Reproduction
[edit]
During the mating season, which varies by species and climate, the male deposits asperm sackat the female's genital opening (namedthelycum). The females can carry several thousand eggs in theirovary,which may then account for as much as one third of the animal's body mass.[55]Krill can have multiple broods in one season, with interbrood intervals lasting on the order of days.[25][56]
Krill employ two types of spawning mechanism.[25]The 57 species of the generaBentheuphausia,Euphausia,Meganyctiphanes,Thysanoessa,andThysanopodaare "broadcast spawners": the female releases the fertilised eggs into the water, where they usually sink, disperse, and are on their own. These species generally hatch in the nauplius 1 stage, but have recently been discovered to hatch sometimes as metanauplius or even as calyptopis stages.[57]The remaining 29 species of the other genera are "sac spawners", where the female carries the eggs with her, attached to the rearmost pairs of thoracopods until they hatch as metanauplii, although some species likeNematoscelis difficilismay hatch as nauplius or pseudometanauplius.[58]
Moulting
[edit]Moulting occurs whenever a specimen outgrows its rigid exoskeleton. Young animals, growing faster, moult more often than older and larger ones. The frequency of moulting varies widely by species and is, even within one species, subject to many external factors such as latitude, water temperature, and food availability. The subtropical speciesNyctiphanes simplex,for instance, has an overall inter-moult period of two to seven days: larvae moult on the average every four days, while juveniles and adults do so, on average, every six days. ForE. superbain the Antarctic sea, inter-moult periods ranging between 9 and 28 days depending on the temperature between −1 and 4 °C (30 and 39 °F) have been observed, and forMeganyctiphanes norvegicain theNorth Seathe inter-moult periods range also from 9 and 28 days but at temperatures between 2.5 and 15 °C (36.5 and 59.0 °F).[59]E. superbais able to reduce its body size when there is not enough food available, moulting also when its exoskeleton becomes too large.[60]Similar shrinkage has also been observed forE. pacifica,a species occurring in the Pacific Ocean from polar to temperate zones, as an adaptation to abnormally high water temperatures. Shrinkage has been postulated for other temperate-zone species of krill as well.[61]
Lifespan
[edit]Some high-latitude species of krill can live for more than six years (e.g.,Euphausia superba); others, such as the mid-latitude speciesEuphausia pacifica,live for only two years.[7]Subtropical or tropical species' longevity is still shorter, e.g.,Nyctiphanes simplex,which usually lives for only six to eight months.[62]
Swarming
[edit]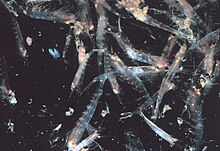
Most krill areswarminganimals; the sizes and densities of such swarms vary by species and region. ForEuphausia superba,swarms reach 10,000 to 60,000 individuals per cubic metre.[63][64]Swarming is a defensive mechanism, confusing smaller predators that would like to pick out individuals. In 2012, Gandomi and Alavi presented what appears to be asuccessful stochastic algorithmfor modelling the behaviour of krill swarms. The algorithm is based on three main factors: "(i) movement induced by the presence of other individuals (ii) foraging activity, and (iii) random diffusion."[65]
Vertical migration
[edit]
Krill typically follow adiurnalvertical migration.It has been assumed that they spend the day at greater depths and rise during the night toward the surface. The deeper they go, the more they reduce their activity,[66]apparently to reduce encounters with predators and to conserve energy. Swimming activity in krill varies with stomach fullness. Sated animals that had been feeding at the surface swim less actively and therefore sink below the mixed layer.[67]As they sink they producefeceswhich employs a role in the Antarcticcarbon cycle.Krill with empty stomachs swim more actively and thus head towards the surface.
Vertical migration may be a 2–3 times daily occurrence. Some species (e.g.,Euphausia superba,E. pacifica,E. hanseni,Pseudeuphausia latifrons,andThysanoessa spinifera) form surface swarms during the day for feeding and reproductive purposes even though such behaviour is dangerous because it makes them extremely vulnerable to predators.[68]
Experimental studies usingArtemia salinaas a model suggest that the vertical migrations of krill several hundreds of metres, in groups tens of metres deep, could collectively create enough downward jets of water to have a significant effect on ocean mixing.[69]
Dense swarms can elicit afeeding frenzyamong fish, birds and mammal predators, especially near the surface. When disturbed, a swarm scatters, and some individuals have even been observed to moult instantly, leaving theexuviabehind as a decoy.[70]
Krill normally swim at a pace of 5–10 cm/s (2–3 body lengths per second),[71]using their swimmerets for propulsion. Their larger migrations are subject to ocean currents. When in danger, they show anescape reactioncalledlobstering—flicking theircaudalstructures, thetelsonand theuropods,they move backwards through the water relatively quickly, achieving speeds in the range of 10 to 27 body lengths per second, which for large krill such asE. superbameans around 0.8 m/s (3 ft/s).[72]Their swimming performance has led many researchers to classify adult krill asmicro-nektoniclife-forms, i.e., small animals capable of individual motion against (weak) currents. Larval forms of krill are generally considered zooplankton.[73]
Biogeochemical cycles
[edit]
The Antarctic krill is an important species in the context ofbiogeochemical cycling[74][42]and in theAntarctic food web.[75][76]It plays a prominent role in the Southern Ocean because of its ability tocycle nutrientsand to feed penguins andbaleenandblue whales.

Human uses
[edit]
Harvesting history
[edit]Krill have been harvested as a food source for humans and domesticated animals since at least the 19th century, and possibly earlier in Japan, where it was known asokiami.Large-scale fishing developed in the late 1960s and early 1970s, and now occurs only in Antarctic waters and in the seas around Japan. Historically, the largest krill fishery nations were Japan and the Soviet Union, or, after the latter's dissolution, Russia andUkraine.[77]The harvest peaked, which in 1983 was about 528,000 tonnes in the Southern Ocean alone (of which the Soviet Union took in 93%), is now managed as a precaution against overfishing.[78]
In 1993, two events caused a decline in krill fishing: Russia exited the industry; and theConvention for the Conservation of Antarctic Marine Living Resources(CCAMLR) defined maximum catch quotas for asustainable exploitationof Antarctic krill. After an October 2011 review, the Commission decided not to change the quota.[79]
The annual Antarctic catch stabilised at around 100,000 tonnes, which is roughly one fiftieth of the CCAMLR catch quota.[80]The main limiting factor was probably high costs along with political and legal issues.[81]The Japanese fishery saturated at some 70,000 tonnes.[82]
Although krill are found worldwide, fishing in Southern Oceans are preferred because the krill are more "catchable" and abundant in these regions. Particularly in Antarctic seas which are considered aspristine,they are considered a "clean product".[77]
In 2018 it was announced that almost every krill fishing company operating in Antarctica will abandon operations in huge areas around the Antarctic Peninsula from 2020, including "buffer zones" around breeding colonies of penguins.[83]
Human consumption
[edit]
Although the totalbiomassof Antarctic krill may be as abundant as 400 million tonnes, the human impact on thiskeystone speciesis growing, with a 39% increase in total fishing yield to 294,000 tonnes over 2010–2014.[80]Major countries involved in krill harvesting areNorway(56% of total catch in 2014), theRepublic of Korea(19%), andChina(18%).[80]
Krill is a rich source ofproteinandomega-3 fatty acidswhich are under development in the early 21st century as human food,dietary supplementsas oil capsules, livestock food, andpet food.[77][79][84]Krill tastes salty with a somewhat stronger fish flavor than shrimp. For mass consumption and commercially prepared products, they must be peeled to remove the inedibleexoskeleton.[84]
In 2011, the USFood and Drug Administrationpublished a letter of no objection for a manufacturedkrill oilproduct to begenerally recognized as safe(GRAS) for human consumption.[85]
Krill (and otherplanktonicshrimp,notablyAcetesspp.) are most widely consumed in Southeast Asia, where it isfermented(with the shells intact) and usually ground finely to makeshrimp paste.It can be stir-fried and eaten paired with white rice or used to addumamiflavors to a wide variety of traditional dishes.[86][87]The liquid from the fermentation process is also harvested asfish sauce.[88]
Bio-inspired robotics
[edit]Krill are agile swimmers in the intermediateReynolds numberregime, in which there are not many solutions for uncrewed underwater robotics, and have inspired robotic platforms to both study their locomotion as well as find design solutions for underwater robots.[89]
See also
[edit]References
[edit]- ^"Euphausiids (Krill)".Government of Canada.Fisheries and Oceans Canada. 6 April 2022.Retrieved18 April2024.
Many different species of euphausiids are found on Canada's east and west coasts.
- ^Crustacea: Euphausiacea - Oxford Academic
- ^"Krill".Online Etymology Dictionary.Retrieved22 June2010.
- ^A. Atkinson; V. Siegel; E.A. Pakhomov; M.J. Jessopp; V. Loeb (2009)."A re-appraisal of the total biomass and annual production of Antarctic krill"(PDF).Deep-Sea Research Part I.56(5): 727–740.Bibcode:2009DSRI...56..727A.doi:10.1016/j.dsr.2008.12.007.
- ^Siegel V (2011). Siegel V (ed.)."Euphausiidae Dana, 1852".World Euphausiacea database.World Register of Marine Species.Retrieved25 November2011.
- ^E. Brinton (1962)."The distribution of Pacific euphausiids".Bull. Scripps Inst. Oceanogr.8(2): 51–270.
- ^abS. Nicol; Y. Endo (1999)."Krill fisheries: Development, management and ecosystem implications".Aquatic Living Resources.12(2): 105–120.doi:10.1016/S0990-7440(99)80020-5.S2CID84158071.
- ^abcdAndreas Maas; Dieter Waloszek (2001)."Larval development ofEuphausia superbaDana, 1852 and a phylogenetic analysis of the Euphausiacea "(PDF).Hydrobiologia.448:143–169.doi:10.1023/A:1017549321961.S2CID32997380.Archived fromthe original(PDF)on 18 July 2011.
- ^Bernadette Casanova (1984). "Phylogénie des Euphausiacés (Crustacés Eucarides)" [Phylogeny of the Euphausiacea (Crustacea: Eucarida)].Bulletin du Muséum National d'Histoire Naturelle(in French).4:1077–1089.
- ^abcBernadette Casanova (2003). "Ordre des Euphausiacea Dana, 1852".Crustaceana.76(9): 1083–1121.doi:10.1163/156854003322753439.JSTOR20105650.
- ^M. Eugenia D'Amato; Gordon W. Harkins; Tulio de Oliveira; Peter R. Teske; Mark J. Gibbons (2008)."Molecular dating and biogeography of the neritic krillNyctiphanes"(PDF).Marine Biology.155(2): 243–247.doi:10.1007/s00227-008-1005-0.S2CID17750015.Archived fromthe original(PDF)on 17 March 2012.Retrieved4 July2010.
- ^abcSimon N. Jarman (2001)."The evolutionary history of krill inferred from nuclear large subunit rDNA sequence analysis".Biological Journal of the Linnean Society.73(2): 199–212.doi:10.1111/j.1095-8312.2001.tb01357.x.
- ^Xin Shen; Haiqing Wang; Minxiao Wang; Bin Liu (2011). "The complete mitochondrial genome sequence ofEuphausia pacifica(Malacostraca: Euphausiacea) reveals a novel gene order and unusual tandem repeats ".Genome.54(11): 911–922.doi:10.1139/g11-053.PMID22017501.
- ^Johan Erik Vesti Boas(1883). "Studien über die Verwandtschaftsbeziehungen der Malacostraken" [Studies on the relationships of the Malacostraca].Morphologisches Jahrbuch(in German).8:485–579.
- ^abRobert Gurney(1942).Larvae of Decapod Crustacea(PDF).Ray Society.
- ^Isabella Gordon(1955)."Systematic position of the Euphausiacea".Nature.176(4489): 934.Bibcode:1955Natur.176..934G.doi:10.1038/176934a0.S2CID4225121.
- ^Trisha Spears, Ronald W. DeBry, Lawrence G. Abele & Katarzyna Chodyl (2005). Boyko, Christopher B. (ed.)."Peracarid monophyly and interordinal phylogeny inferred from nuclear small-subunit ribosomal DNA sequences (Crustacea: Malacostraca: Peracarida)"(PDF).Proceedings of the Biological Society of Washington.118(1): 117–157.doi:10.2988/0006-324X(2005)118[117:PMAIPI]2.0.CO;2.S2CID85557065.
{{cite journal}}:CS1 maint: multiple names: authors list (link) - ^K. Meland; E. Willassen (2007). "The disunity of" Mysidacea "(Crustacea)".Molecular Phylogenetics and Evolution.44(3): 1083–1104.CiteSeerX10.1.1.653.5935.doi:10.1016/j.ympev.2007.02.009.PMID17398121.
- ^Frederick R. Schram(1986).Crustacea.Oxford University Press.ISBN978-0-19-503742-5.
- ^J. J. Torres; J. J. Childress (1985). "Respiration and chemical composition of the bathypelagic euphausiidBentheuphausia amblyops".Marine Biology.87(3): 267–272.doi:10.1007/BF00397804.S2CID84486097.
- ^Volker Siegel (2011)."ThysanoessaBrandt, 1851 ".WoRMS.World Register of Marine Species.Retrieved18 June2011.
- ^abD'Amato, M.E.et al.:"Molecular dating and biogeography of the neritic krillNyctiphanesArchived17 March 2012 at theWayback Machine",inMarine Biology vol. 155, no. 2,pp. 243–247, August 2008.
- ^Volker Siegel (2011). V. Siegel (ed.)."NyctiphanesSars, 1883 ".World Euphausiacea database.World Register of Marine Species.Retrieved18 June2011.
- ^abcJ. Mauchline; L. R. Fisher (1969).The Biology of Euphausiids.Advances in Marine Biology. Vol. 7.Academic Press.ISBN978-7-7708-3615-2.
- ^abcJaime Gómez-Gutiérrez; Carlos J. Robinson (2005)."Embryonic, early larval development time, hatching mechanism and interbrood period of the sac-spawning euphausiidNyctiphanes simplexHansen ".Journal of Plankton Research.27(3): 279–295.doi:10.1093/plankt/fbi003.
- ^S. N. Jarman; N. G. Elliott; S. Nicol; A. McMinn (2002)."Genetic differentiation in the Antarctic coastal krillEuphausia crystallorophias".Heredity.88(4): 280–287.doi:10.1038/sj.hdy.6800041.PMID11920136.
- ^R. Escribano; V. Marin; C. Irribarren (2000)."Distribution ofEuphausia mucronataat the upwelling area of Peninsula Mejillones, northern Chile: the influence of the oxygen minimum layer ".Scientia Marina.64(1): 69–77.doi:10.3989/scimar.2000.64n169.
- ^P. Brueggeman."Euphausia crystallorophias".Underwater Field Guide to Ross Island & McMurdo Sound, Antarctica.University of California, San Diego.
- ^"Krill,Euphausia superba".MarineBio.org.Retrieved25 February2009.
- ^J. A. Kirkwood (1984). "A Guide to the Euphausiacea of the Southern Ocean".ANARE Research Notes.1:1–45.
- ^Bianchi, Daniele; Mislan, K.A.S. (January 2016)."Global patterns of diel vertical migration times and velocities from acoustic data".Limnology and Oceanography.61(1).doi:10.1002/lno.10219.
- ^A. Sala; M. Azzali; A. Russo (2002)."Krill of the Ross Sea: distribution, abundance and demography ofEuphausia superbaandEuphausia crystallorophiasduring the Italian Antarctic Expedition (January–February 2000) ".Scientia Marina.66(2): 123–133.doi:10.3989/scimar.2002.66n2123.
- ^G. W. Hosie; M. Fukuchi; S. Kawaguchi (2003)."Development of the Southern Ocean Continuous Plankton Recorder survey"(PDF).Progress in Oceanography.58(2–4): 263–283.Bibcode:2003PrOce..58..263H.doi:10.1016/j.pocean.2003.08.007.[permanent dead link]
- ^E. Gaten."Meganyctiphanes norvegica".University of Leicester.Archived fromthe originalon 1 July 2009.Retrieved25 February2009.
- ^E. Brinton (1953). "Thysanopoda spinicauda,a new bathypelagic giant euphausiid crustacean, with comparative notes onT. cornutaandT. egregia".Journal of the Washington Academy of Sciences.43:408–412.
- ^"Euphausiacea".Tasmanian Aquaculture & Fisheries Institute. Archived fromthe originalon 30 September 2009.Retrieved6 June2010.
- ^O. Shimomura (1995). "The roles of the two highly unstable components F and P involved in the bioluminescence of euphausiid shrimps".Journal of Bioluminescence and Chemiluminescence.10(2): 91–101.doi:10.1002/bio.1170100205.PMID7676855.
- ^J. C. Dunlap; J. W. Hastings; O. Shimomura (1980)."Crossreactivity between the light-emitting systems of distantly related organisms: novel type of light-emitting compound".Proceedings of the National Academy of Sciences.77(3): 1394–1397.Bibcode:1980PNAS...77.1394D.doi:10.1073/pnas.77.3.1394.JSTOR8463.PMC348501.PMID16592787.
- ^P. J. Herring; E. A. Widder (2001)."Bioluminescence in Plankton and Nekton".In J. H. Steele; S. A. Thorpe; K. K. Turekian (eds.).Encyclopedia of Ocean Science.Vol. 1.Academic Press,San Diego. pp.308–317.ISBN978-0-12-227430-5.
- ^S. M. Lindsay; M. I. Latz (1999).Experimental evidence for luminescent countershading by some euphausiid crustaceans.American Society of Limnology and Oceanography (ASLO) Aquatic Sciences Meeting. Santa Fe.
- ^Sönke Johnsen (2005)."The Red and the Black: bioluminescence and the color of animals in the deep sea"(PDF).Integrative and Comparative Biology.4(2): 234–246.doi:10.1093/icb/45.2.234.PMID21676767.S2CID247718.Archived fromthe original(PDF)on 2 October 2005.
- ^abcdCavan, E.L., Belcher, A., Atkinson, A., Hill, S.L., Kawaguchi, S., McCormack, S., Meyer, B., Nicol, S., Ratnarajah, L., Schmidt, K. and Steinberg, D.K. (2019) "The importance of Antarctic krill in biogeochemical cycles".Nature communications,10(1): 1–13.doi:10.1038/s41467-019-12668-7.
 Material was copied from this source, which is available under aCreative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under aCreative Commons Attribution 4.0 International License.
- ^G. C. Cripps; A. Atkinson (2000). "Fatty acid composition as an indicator of carnivory in Antarctic krill,Euphausia superba".Canadian Journal of Fisheries and Aquatic Sciences.57(S3): 31–37.doi:10.1139/f00-167.
- ^abOlav Saether; Trond Erling Ellingsen; Viggo Mohr (1986)."Lipids of North Atlantic krill"(PDF).Journal of Lipid Research.27(3): 274–285.PMID3734626.
- ^M. J. Schramm (10 October 2007)."Tiny Krill: Giants in Marine Food Chain".NOAA National Marine Sanctuary Program.Retrieved4 June2010.
- ^J. Weier (1999)."Changing currents color the Bering Sea a new shade of blue".NOAAEarth Observatory.Retrieved15 June2005.
- ^R. D. Brodeur; G. H. Kruse; P. A. Livingston; G. Walters; J. Ianelli; G. L. Swartzman; M. Stepanenko; T. Wyllie-Echeverria (1998).Draft Report of the FOCI International Workshop on Recent Conditions in the Bering Sea.NOAA.pp. 22–26.
- ^J. Roach (17 July 2003)."Scientists discover mystery krill killer".National Geographic News.Archived fromthe originalon 24 July 2003.
- ^J. Gómez-Gutiérrez; W. T. Peterson; A. de Robertis; R. D. Brodeur (2003). "Mass mortality of krill caused by parasitoid ciliates".Science.301(5631): 339.doi:10.1126/science.1085164.PMID12869754.S2CID28471713.
- ^J. D. Shields; J. Gómez-Gutiérrez (1996). "Oculophryxus bicaulis,a new genus and species of dajid isopod parasitic on the euphausiidStylocheiron affineHansen ".International Journal for Parasitology.26(3): 261–268.doi:10.1016/0020-7519(95)00126-3.PMID8786215.
- ^Rusty Dornin (6 July 1997)."Antarctic krill populations decreasing".CNN.Retrieved18 June2011.
- ^Dawson, Amanda L; Kawaguchi, So; King, Catherine K; Townsend, Kathy A; King, Robert; Huston, Wilhelmina M; Bengtson Nash, Susan M (2018)."Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill".Nature Communications.9(1): 1001.Bibcode:2018NatCo...9.1001D.doi:10.1038/s41467-018-03465-9.PMC5843626.PMID29520086.
- ^M. D. Knight (1984)."Variation in larval morphogenesis within the Southern California Bight population ofEuphausia pacificafrom Winter through Summer, 1977–1978 "(PDF).CalCOFI Report.XXV.Archived fromthe original(PDF)on 3 August 2019.Retrieved5 November2017.
- ^"Euphausia superba".Species factsheet.Food and Agriculture Organization.Retrieved4 June2010.
- ^R. M. Ross; L. B. Quetin (1986). "How productive are Antarctic krill?".BioScience.36(4): 264–269.doi:10.2307/1310217.JSTOR1310217.
- ^Janine Cuzin-Roudy (2000). "Seasonal reproduction, multiple spawning, and fecundity in northern krill,Meganyctiphanes norvegica,and Antarctic krill,Euphausia superba".Canadian Journal of Fisheries and Aquatic Sciences.57(S3): 6–15.doi:10.1139/f00-165.
- ^J. Gómez-Gutiérrez (2002)."Hatching mechanism and delayed hatching of the eggs of three broadcast spawning euphausiid species under laboratory conditions".Journal of Plankton Research.24(12): 1265–1276.doi:10.1093/plankt/24.12.1265.
- ^E. Brinton; M. D. Ohman; A. W. Townsend; M. D. Knight; A. L. Bridgeman (2000).Euphausiids of the World Ocean.World Biodiversity Database CD-ROM Series,Springer Verlag.ISBN978-3-540-14673-5.Archived fromthe originalon 26 February 2012.Retrieved4 December2009.
- ^F. Buchholz (2003). "Experiments on the physiology of Southern and Northern krill,Euphausia superbaandMeganyctiphanes norvegica,with emphasis on moult and growth – a review ".Marine and Freshwater Behaviour and Physiology.36(4): 229–247.Bibcode:2003MFBP...36..229B.doi:10.1080/10236240310001623376.S2CID85121989.
- ^H.-C. Shin; S. Nicol (2002)."Using the relationship between eye diameter and body length to detect the effects of long-term starvation on Antarctic krillEuphausia superba".Marine Ecology Progress Series.239:157–167.Bibcode:2002MEPS..239..157S.doi:10.3354/meps239157.
- ^B. Marinovic; M. Mangel (1999)."Krill can shrink as an ecological adaptation to temporarily unfavourable environments"(PDF).Ecology Letters.2:338–343.
- ^J. G. Gómez (1995)."Distribution patterns, abundance and population dynamics of the euphausiidsNyctiphanes simplexandEuphausia eximiaoff the west coast of Baja California, Mexico "(PDF).Marine Ecology Progress Series.119:63–76.Bibcode:1995MEPS..119...63G.doi:10.3354/meps119063.
- ^U. Kils; P. Marshall (1995). "Der Krill, wie er schwimmt und frisst – neue Einsichten mit neuen Methoden ("The Antarctic krill – how it swims and feeds – new insights with new methods")". In I. Hempel; G. Hempel (eds.).Biologie der Polarmeere – Erlebnisse und Ergebnisse (Biology of the Polar Oceans Experiences and Results).Fischer Verlag.pp. 201–210.ISBN978-3-334-60950-7.
- ^R. Piper (2007).Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals.Greenwood Press.ISBN978-0-313-33922-6.
- ^Gandomi, A.H.; Alavi, A.H. (2012). "Krill Herd: A New Bio-Inspired Optimization Algorithm".Communications in Nonlinear Science and Numerical Simulation.17(12): 4831–4845.Bibcode:2012CNSNS..17.4831G.doi:10.1016/j.cnsns.2012.05.010.
- ^J. S. Jaffe; M. D. Ohmann; A. de Robertis (1999)."Sonar estimates of daytime activity levels ofEuphausia pacificain Saanich Inlet "(PDF).Canadian Journal of Fisheries and Aquatic Sciences.56(11): 2000–2010.doi:10.1139/cjfas-56-11-2000.S2CID228567512.Archived fromthe original(PDF)on 20 July 2011.
- ^Geraint A. Tarling; Magnus L. Johnson (2006)."Satiation gives krill that sinking feeling".Current Biology.16(3): 83–84.doi:10.1016/j.cub.2006.01.044.PMID16461267.
- ^Dan Howard (2001)."Krill"(PDF).In Herman A. Karl; John L. Chin; Edward Ueber; Peter H. Stauffer; James W. Hendley II (eds.).Beyond the Golden Gate – Oceanography, Geology, Biology, and Environmental Issues in the Gulf of the Farallones.United States Geological Survey.pp. 133–140. Circular 1198.Retrieved8 October2011.
- ^Wishart, Skye (July–August 2018)."The krill effect".New Zealand Geographic(152): 24.
- ^D. Howard."Krill in Cordell Bank National Marine Sanctuary".National Oceanic and Atmospheric Administration.Retrieved15 June2005.
- ^David A. Demer; Stéphane G. Conti (2005)."New target-strength model indicates more krill in the Southern Ocean".ICES Journal of Marine Science.62(1): 25–32.doi:10.1016/j.icesjms.2004.07.027.
- ^U. Kils (1982)."Swimming behavior, swimming performance and energy balance of Antarctic krillEuphausia superba".BIOMASS Scientific Series 3, BIOMASS Research Series:1–122. Archived fromthe originalon 2 June 2020.Retrieved11 November2017.
- ^S. Nicol; Y. Endo (1997)."Krill Fisheries of the World".FAO Fisheries Technical Paper.367.
- ^Ratnarajah, L., Bowie, A.R., Lannuzel, D., Meiners, K.M. and Nicol, S. (2014) "The biogeochemical role of baleen whales and krill in Southern Ocean nutrient cycling".PLOS ONE,9(12): e114067.doi:10.1371/journal.pone.0114067
- ^Hopkins, T.L., Ainley, D.G., Torres, J.J., Lancraft, T.M., 1993. Trophic structure in open waters of the Marginal Ice Zone in the Scotia Weddell Confluence region during spring (1983). Polar Biology 13, 389–397.
- ^Lancraft, T.M., Relsenbichler, K.R., Robinson, B.H., Hopkins, T.L., Torres, J.J., 2004. A krill-dominated micronekton and macrozooplankton community in Croker Passage, Antarctica with an estimate of fish predation. Deep-Sea Research II 51, 2247–2260.
- ^abcGrossman, Elizabeth (14 July 2015)."Scientists consider whether krill need to be protected from human over-hunting".Public Radio International (PRI).Retrieved1 April2017.
- ^"Krill fisheries and sustainability: Antarctic krill (Euphausia superba)".Commission for the Conservation of Antarctic Marine Living Resources. 23 April 2015.Retrieved1 April2017.
- ^abSchiermeier, Q (2010)."Ecologists fear Antarctic krill crisis".Nature.467(7311): 15.doi:10.1038/467015a.PMID20811427.
- ^abc"Krill – biology, ecology and fishing".Commission for the Conservation of Antarctic Marine Living Resources. 28 April 2015.Retrieved1 April2017.
- ^Minturn J. Wright (1987)."The Ownership of Antarctica, its Living and Mineral Resources".Journal of Law and the Environment.4(2): 49–78.
- ^S. Nicol; J. Foster (2003)."Recent trends in the fishery for Antarctic krill".Aquatic Living Resources.16:42–45.doi:10.1016/S0990-7440(03)00004-4.
- ^Josh, Gabbatiss (10 July 2018)."Krill fishing industry backs massive Antarctic ocean sanctuary to protect penguins, seals and whales".The Independent.Retrieved10 July2018.
- ^ab"Why krill?".Southwest Fisheries Science Center, US National Oceanic and Atmospheric Administration. 22 November 2016.Retrieved1 April2017.
- ^Cheeseman MA (22 July 2011)."Krill oil: Agency Response Letter GRAS Notice No. GRN 000371".US FDA.Retrieved3 June2015.
- ^Omori, M. (1978). "Zooplankton fisheries of the world: A review".Marine Biology.48(3): 199–205.doi:10.1007/BF00397145.S2CID86540101.
- ^Pongsetkul, Jaksuma; Benjakul, Soottawat; Sampavapol, Punnanee; Osako, Kazufumi; Faithong, Nandhsha (17 September 2014)."Chemical composition and physical properties of salted shrimp paste (Kapi) produced in Thailand".International Aquatic Research.6(3): 155–166.doi:10.1007/s40071-014-0076-4.
- ^Abe, Kenji; Suzuki, Kenji; Hashimoto, Kanehisa (1979)."Utilization of Krill as a Fish Sauce Material".Nippon Suisan Gakkaishi.45(8): 1013–1017.doi:10.2331/suisan.45.1013.
- ^Oliveira Santos, Sara; Tack, Nils; Su, Yunxing; Cuenca-Jimenez, Francisco; Morales-Lopez, Oscar; Gomez-Valdez, P. Antonio; M Wilhelmus, Monica (13 June 2023)."Pleobot: a modular robotic solution for metachronal swimming".Scientific Reports.13(1): 9574.Bibcode:2023NatSR..13.9574O.doi:10.1038/s41598-023-36185-2.PMC10264458.PMID37311777.
Further reading
[edit]- Boden, Brian P.;Johnson, Martin W.;Brinton, Edward:"Euphausiacea (Crustacea) of the North Pacific".Bulletin of the Scripps Institution of Oceanography.Volume 6 Number 8, 1955.
- Brinton, Edward:"Euphausiids of Southeast Asian waters".Naga Reportvolume 4, part 5. La Jolla: University of California, Scripps Institution of Oceanography, 1975.
- Conway, D. V. P.; White, R. G.; Hugues-Dit-Ciles, J.; Galienne, C. P.; Robins, D. B.:Guide to the coastal and surface zooplankton of the South-Western Indian OceanArchived23 October 2012 at theWayback Machine,OrderEuphausiacea,Occasional Publication of theMarine Biological Association of the United KingdomNo. 15, Plymouth, UK, 2003.
- Everson, I. (ed.):Krill: biology, ecology and fisheries.Oxford, Blackwell Science; 2000.ISBN0-632-05565-0.
- Hamner, William M. (May 1984). "Krill — Untapped Bounty From the Sea?".National Geographic.Vol. 165, no. 5. pp. 626–642.ISSN0027-9358.OCLC643483454.
- Mauchline, J.:Euphausiacea:AdultsArchived15 May 2011 at theWayback Machine,Conseil International pour l'Exploration de la Mer, 1971. Identification sheets for adult krill with many line drawings.PDFfile, 2Mb.
- Mauchline, J.:Euphausiacea:LarvaeArchived19 April 2012 at theWayback Machine,Conseil International pour l'Exploration de la Mer, 1971. Identification sheets for larval stages of krill with many line drawings. PDF file, 3 Mb.
- Tett, P.:The biology of Euphausiids,lecture notes from a2003 course in Marine BiologyfromNapier University.
- Tett, P.:Bioluminescence,lecture notes from the 1999/2000 edition of that same course.
External links
[edit]- Webcam of Krill Aquarium at Australian Antarctic Division
- 'Antarctic Energies'animation by Lisa Roberts




