Fatty acid metabolism
Fatty acid metabolismconsists of variousmetabolicprocesses involving or closely related tofatty acids,a family of molecules classified within thelipidmacronutrientcategory. These processes can mainly be divided into (1)catabolicprocesses that generate energy and (2)anabolicprocesses where they serve as building blocks for other compounds.[1]
In catabolism, fatty acids are metabolized to produce energy, mainly in the form ofadenosine triphosphate(ATP). When compared to other macronutrient classes (carbohydrates and protein), fatty acids yield the most ATP on an energy per gram basis, when they are completely oxidized to CO2and water bybeta oxidationand thecitric acid cycle.[2]Fatty acids (mainly in the form oftriglycerides) are therefore the foremost storage form of fuel in most animals, and to a lesser extent in plants.
In anabolism, intact fatty acids are important precursors to triglycerides, phospholipids, second messengers, hormones andketone bodies.For example,phospholipidsform thephospholipid bilayersout of which all the membranes of the cell are constructed from fatty acids. Phospholipids comprise the plasma membrane and other membranes that enclose all theorganelleswithin the cells, such as thenucleus,themitochondria,endoplasmic reticulum,and theGolgi apparatus.In another type of anabolism, fatty acids are modified to form other compounds such assecond messengersandlocal hormones.Theprostaglandinsmade fromarachidonic acidstored in the cell membrane are probably the best-known of these local hormones.
Fatty acid catabolism[edit]
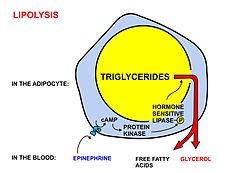


Fatty acids are stored astriglyceridesin the fat depots ofadipose tissue.Between meals they are released as follows:
- Lipolysis,the removal of the fatty acid chains from the glycerol to which they are bound in their storage form as triglycerides (or fats), is carried out bylipases.These lipases are activated by highepinephrineandglucagonlevels in the blood (ornorepinephrinesecreted bysympathetic nervesin adipose tissue), caused by declining bloodglucoselevels after meals, which simultaneously lowers theinsulinlevel in the blood.[1]
- Once freed fromglycerol,the free fatty acids enter the blood, which transports them, attached to plasmaalbumin,throughout the body.[4]
- Long-chain free fatty acids enter metabolizing cells (i.e. most living cells in the body exceptred blood cellsandneuronsin thecentral nervous system) through specifictransport proteins,such as theSLC27family fatty acid transport protein.[5][6]Red blood cells do not containmitochondriaand are therefore incapable of metabolizing fatty acids; the tissues of the central nervous system cannot use fatty acids, despite containing mitochondria, because long-chain fatty acids (as opposed to medium-chain fatty acids[7][8]) cannot cross theblood-brain barrier[9]into theinterstitial fluidsthat bathe these cells.
- Once inside the cell,long-chain-fatty-acid—CoA ligasecatalyzes the reaction between a fatty acid molecule withATP(which is broken down toAMPand inorganic pyrophosphate) to give a fatty acyl-adenylate, which then reacts with freecoenzyme Ato give a fattyacyl-CoAmolecule.
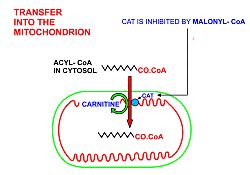
- In order for the acyl-CoA to enter the mitochondrion the carnitine shuttle is used:[10][11][12]
- Acyl-CoA is transferred to the hydroxyl group of carnitine bycarnitine palmitoyltransferase I,located on the cytosolic faces of theouterandinner mitochondrial membranes.
- Acyl-carnitine is shuttled inside by acarnitine-acylcarnitine translocase,as a carnitine is shuttled outside.
- Acyl-carnitine is converted back to acyl-CoA bycarnitine palmitoyltransferase II,located on the interior face of theinner mitochondrial membrane.The liberated carnitine is shuttled back to the cytosol, as an acyl-CoA is shuttled into the mitochondrial matrix.
- Beta oxidation,in the mitochondrial matrix, then cuts the long carbon chains of the fatty acids (in the form of acyl-CoA molecules) into a series of two-carbon (acetate) units, which, combined withco-enzyme A,form molecules ofacetyl CoA,which condense withoxaloacetateto formcitrateat the "beginning" of thecitric acid cycle.[2]It is convenient to think of this reaction as marking the "starting point" of the cycle, as this is when fuel - acetyl-CoA - is added to the cycle, which will be dissipated as CO2and H2O with the release of a substantial quantity of energy captured in the form ofATP,during the course of each turn of the cycle and subsequentoxidative phosphorylation.
- Briefly, the steps in beta oxidation are as follows:[2]
- Dehydrogenation byacyl-CoA dehydrogenase,yielding 1FADH2
- Hydration byenoyl-CoA hydratase
- Dehydrogenation by3-hydroxyacyl-CoA dehydrogenase,yielding 1NADH + H+
- Cleavage bythiolase,yielding 1acetyl-CoAand a fatty acid that has now been shortened by 2 carbons (forming a new, shortenedacyl-CoA)
- This beta oxidation reaction is repeated until the fatty acid has been completely reduced toacetyl-CoAor, in, the case of fatty acids with odd numbers of carbon atoms,acetyl-CoAand 1 molecule ofpropionyl-CoAper molecule of fatty acid. Each beta oxidative cut of the acyl-CoA molecule eventually yields 5ATPmolecules in oxidative phosphorylation.[13][14]
- The acetyl-CoA produced by beta oxidation enters thecitric acid cyclein the mitochondrion by combining withoxaloacetateto formcitrate.Coupled to oxidative phosphorylation this results in the complete combustion of the acetyl-CoA to CO2and water. The energy released in this process is captured in the form of 1GTPand 11ATPmolecules per acetyl-CoA molecule oxidized.[2][10]This is the fate of acetyl-CoA wherever beta oxidation of fatty acids occurs, except under certain circumstances in theliver.
- The propionyl-CoA is later converted intosuccinyl-CoAthroughbiotin-dependantpropionyl-CoA carboxylase(PCC) andVitamin B12-dependantmethylmalonyl-CoA mutase(MCM), sequentially.[15][16]Succinyl-CoA is first converted to malate, and then to pyruvate where it is then transported to the matrix to enter the citric acid cycle.
In the liver oxaloacetate can be wholly or partially diverted into thegluconeogenic pathwayduring fasting, starvation, a low carbohydrate diet, prolonged strenuous exercise, and in uncontrolledtype 1 diabetes mellitus.Under these circumstances, oxaloacetate is hydrogenated tomalate,which is then removed from the mitochondria of the liver cells to be converted intoglucosein the cytoplasm of the liver cells, from where it is released into the blood.[10]In the liver, therefore, oxaloacetate is unavailable for condensation with acetyl-CoA when significant gluconeogenesis has been stimulated by low (or absent)insulinand highglucagonconcentrations in the blood. Under these conditions, acetyl-CoA is diverted to the formation ofacetoacetateandbeta-hydroxybutyrate.[10]Acetoacetate, beta-hydroxybutyrate, and their spontaneous breakdown product,acetone,are frequently, but confusingly, known asketone bodies(as they are not "bodies" at all, but water-soluble chemical substances). The ketones are released by the liver into the blood. All cells with mitochondria can take up ketones from the blood and reconvert them into acetyl-CoA, which can then be used as fuel in their citric acid cycles, as no other tissue can divert its oxaloacetate into the gluconeogenic pathway in the way that this can occur in the liver. Unlike free fatty acids, ketones can cross theblood–brain barrierand are therefore available as fuel for the cells of thecentral nervous system,acting as a substitute for glucose, on which these cells normally survive.[10]The occurrence of high levels of ketones in the blood during starvation, a low carbohydrate diet, prolonged heavy exercise, or uncontrolled type 1 diabetes mellitus is known asketosis,and, in its extreme form, in out-of-control type 1 diabetes mellitus, asketoacidosis.
- The glycerol released by lipase action isphosphorylatedbyglycerol kinasein the liver (the only tissue in which this reaction can occur), and the resultingglycerol 3-phosphateis oxidized todihydroxyacetone phosphate.The glycolytic enzymetriose phosphate isomeraseconverts this compound toglyceraldehyde 3-phosphate,which is oxidized viaglycolysis,or converted to glucose viagluconeogenesis.
Fatty acids as an energy source[edit]

Fatty acids, stored as triglycerides in an organism, are a concentratedsource of energybecause they contain little oxygen and areanhydrous.The energy yield from agramof fatty acids is approximately 9kcal(37 kJ), much higher than the 4 kcal (17 kJ) for carbohydrates. Since thehydrocarbonportion of fatty acids ishydrophobic,thesemoleculescan be stored in a relativelyanhydrous(water-free) environment. Carbohydrates, on the other hand, are more highly hydrated. For example, 1 g ofglycogenbinds approximately 2 g ofwater,which translates to 1.33 kcal/g (4 kcal/3 g). This means that fatty acids can hold more than six times the amount of energy per unit of stored mass. Put another way, if the human body relied on carbohydrates to store energy, then a person would need to carry 31 kg (67.5lb) of hydrated glycogen to have the energy equivalent to 4.6 kg (10 lb) offat.[10]
Hibernatinganimals provide a good example for utilization of fat reserves as fuel. For example, bears hibernate for about 7 months, and during this entire period, the energy is derived from degradation of fat stores. Migrating birds similarly build up large fat reserves before embarking on their intercontinental journeys.[17]
The fat stores of young adult humans average between about 10–20 kg, but vary greatly depending on gender and individual disposition.[18]By contrast, the human body stores only about 400 g ofglycogen,of which 300 g is locked inside the skeletal muscles and is unavailable to the body as a whole. The 100 g or so of glycogen stored in the liver is depleted within one day of starvation.[10]Thereafter the glucose that is released into the blood by the liver for general use by the body tissues has to be synthesized fromthe glucogenic amino acidsand a few othergluconeogenic substrates,which do not include fatty acids.[1]Nonetheless, lipolysis releases glycerol which can enter the pathway of gluconeogenesis.
Carbohydrate synthesis from glycerol and fatty acids[edit]
Fatty acids are broken down to acetyl-CoA by means of beta oxidation inside the mitochondria, whereasfatty acids are synthesizedfrom acetyl-CoA outside the mitochondria, in the cytosol. The two pathways are distinct, not only in where they occur, but also in the reactions that occur, and the substrates that are used. The two pathways are mutually inhibitory, preventing the acetyl-CoA produced by beta-oxidation from entering the synthetic pathway via theacetyl-CoA carboxylasereaction.[1]It can also not be converted topyruvateas thepyruvate dehydrogenase complexreaction is irreversible.[10]Instead the acetyl-CoA produced by the beta-oxidation of fatty acids condenses withoxaloacetate,to enter thecitric acid cycle.During each turn of the cycle, two carbon atoms leave the cycle as CO2in the decarboxylation reactions catalyzed byisocitrate dehydrogenaseandalpha-ketoglutarate dehydrogenase.Thus each turn of the citric acid cycle oxidizes an acetyl-CoA unit while regenerating the oxaloacetate molecule with which the acetyl-CoA had originally combined to form citric acid. The decarboxylation reactions occur beforemalateis formed in the cycle.[1]Only plants possess the enzymes to convert acetyl-CoA into oxaloacetate from which malate can be formed to ultimately be converted to glucose.[1]
However, acetyl-CoA can be converted to acetoacetate, which can decarboxylate toacetone(either spontaneously, or catalyzed byacetoacetate decarboxylase). It can then be further metabolized to isopropanol which is excreted in breath/urine, or byCYP2E1intohydroxyacetone(acetol). Acetol can be converted topropylene glycol.This converts topyruvate(by two alternative enzymes), orpropionaldehyde,or toL-lactaldehydethenL-lactate(the common lactate isomer).[19][20][21]Another pathway turns acetol tomethylglyoxal,then topyruvate,or toD-lactaldehyde(viaS-D-lactoyl-glutathione or otherwise) thenD-lactate.[20][22][23]D-lactate metabolism (to glucose) is slow or impaired in humans, so most of the D-lactate is excreted in the urine; thusD-lactate derived from acetone can contribute significantly to the metabolic acidosis associated with ketosis or isopropanol intoxication.[20]L-Lactate can complete the net conversion of fatty acids into glucose. The first experiment to show conversion of acetone to glucose was carried out in 1951. This, and further experiments used carbonisotopic labelling.[21]Up to 11% of the glucose can be derived from acetone during starvation in humans.[21]
The glycerol released into the blood during thelipolysisof triglycerides in adipose tissue can only be taken up by the liver. Here it is converted intoglycerol 3-phosphateby the action ofglycerol kinasewhich hydrolyzes one molecule ofATPper glycerol molecule which is phosphorylated. Glycerol 3-phosphate is then oxidized todihydroxyacetone phosphate,which is, in turn, converted intoglyceraldehyde 3-phosphateby the enzymetriose phosphate isomerase.From here the three carbon atoms of the original glycerol can be oxidized viaglycolysis,or converted to glucose viagluconeogenesis.[10]
Other functions and uses of fatty acids[edit]
Intracellular signaling[edit]

Fatty acids are an integral part of the phospholipids that make up the bulk of theplasma membranes,or cell membranes, of cells. These phospholipids can be cleaved intodiacylglycerol(DAG) andinositol trisphosphate(IP3) throughhydrolysisof the phospholipid,phosphatidylinositol 4,5-bisphosphate(PIP2), by the cell membrane bound enzymephospholipase C(PLC).[24]
Eicosanoid paracrine hormones[edit]


One product of fatty acid metabolism are theprostaglandins,compounds having diversehormone-like effects in animals. Prostaglandins have been found in almost everytissuein humans and other animals. They areenzymaticallyderived from arachidonic acid, a 20-carbon polyunsaturated fatty acid. Every prostaglandin therefore contains 20carbonatoms, including a5-carbon ring.They are a subclass ofeicosanoidsand form theprostanoidclass of fatty acid derivatives.[25]
The prostaglandins are synthesized in the cell membrane by the cleavage of arachidonate from the phospholipids that make up the membrane. This is catalyzed either byphospholipase A2acting directly on a membrane phospholipid, or by a lipase acting on DAG (diacyl-glycerol). The arachidonate is then acted upon by thecyclooxygenasecomponent ofprostaglandin synthase.This forms acyclopentanering roughly in the middle of the fatty acid chain. The reaction also adds 4 oxygen atoms derived from two molecules of O2.The resulting molecule is prostaglandin G2,which is converted by the hydroperoxidase component of the enzyme complex into prostaglandin H2.This highly unstable compound is rapidly transformed into other prostaglandins, prostacyclin and thromboxanes.[25]These are then released into the interstitial fluids surrounding the cells that have manufactured the eicosanoid hormone.
If arachidonate is acted upon by alipoxygenaseinstead of cyclooxygenase,hydroxyeicosatetraenoic acidsandleukotrienesare formed. They also act as local hormones.
Prostaglandins have two derivatives:prostacyclinsandthromboxanes.Prostacyclins are powerful locally actingvasodilatorsand inhibit the aggregation of bloodplatelets.Through their role in vasodilation, prostacyclins are also involved ininflammation.They are synthesized in the walls of blood vessels and serve the physiological function of preventing needless clot formation, as well as regulating the contraction ofsmooth muscletissue.[26]Conversely, thromboxanes (produced by platelet cells) arevasoconstrictorsand facilitate platelet aggregation. Their name comes from their role in clot formation (thrombosis).
Dietary sources of fatty acids, their digestion, absorption, transport in the blood and storage[edit]



A significant proportion of the fatty acids in the body are obtained from the diet, in the form of triglycerides of either animal or plant origin. The fatty acids in the fats obtained from land animals tend to be saturated, whereas the fatty acids in the triglycerides of fish and plants are often polyunsaturated and therefore present as oils.
Thesetriglyceridescannot be absorbed by theintestine.[27]They are broken down intomono-anddi-glyceridesplus free fatty acids (but no free glycerol) bypancreatic lipase,which forms a 1:1 complex with a protein calledcolipase(also a constituent of pancreatic juice), which is necessary for its activity. The activated complex can work only at a water-fat interface. Therefore, it is essential that fats are firstemulsifiedbybile saltsfor optimal activity of these enzymes.[28]The digestion products consisting of a mixture of tri-, di- and monoglycerides and free fatty acids, which, together with the other fat soluble contents of the diet (e.g. the fat soluble vitamins and cholesterol) and bile salts form mixedmicelles,in the watery duodenal contents (see diagrams on the right).[27][29]
The contents of these micelles (but not the bile salts) enter theenterocytes(epithelial cells lining the small intestine) where they are resynthesized into triglycerides, and packaged intochylomicronswhich are released into thelacteals(the capillaries of thelymph systemof the intestines).[30]These lacteals drain into thethoracic ductwhich empties into the venous blood at the junction of the left jugular and left subclavian veins on the lower left hand side of the neck. This means that the fat-soluble products of digestion are discharged directly into the general circulation, without first passing through the liver, unlike all other digestion products. The reason for this peculiarity is unknown.[31]

The chylomicrons circulate throughout the body, giving theblood plasmaa milky or creamy appearance after a fatty meal.[citation needed]Lipoprotein lipaseon theendothelial surfacesof the capillaries, especially inadipose tissue,but to a lesser extent also in other tissues, partially digests the chylomicrons into free fatty acids, glycerol and chylomicron remnants. The fatty acids are absorbed by the adipocytes[citation needed],but the glycerol andchylomicron remnantsremain in the blood plasma, ultimately to be removed from the circulation by the liver. The free fatty acids released by the digestion of the chylomicrons are absorbed by the adipocytes[citation needed],where they are resynthesized into triglycerides using glycerol derived from glucose in theglycolytic pathway[citation needed].These triglycerides are stored, until needed for the fuel requirements of other tissues, in the fat droplet of theadipocyte.
Theliverabsorbs a proportion of the glucose from the blood in theportal veincoming from the intestines. After the liver has replenished itsglycogenstores (which amount to only about 100 g of glycogen when full) much of the rest of the glucose is converted into fatty acids as described below. These fatty acids are combined with glycerol to form triglycerides which are packaged into droplets very similar to chylomicrons, but known asvery low-density lipoproteins(VLDL). These VLDL droplets are processed in exactly the same manner as chylomicrons, except that the VLDL remnant is known as anintermediate-density lipoprotein(IDL), which is capable of scavenging cholesterol from the blood. This converts IDL intolow-density lipoprotein(LDL), which is taken up by cells that require cholesterol for incorporation into their cell membranes or for synthetic purposes (e.g. the formation of thesteroid hormones). The remainder of the LDLs is removed by the liver.[32]
Adipose tissueand lactatingmammary glandsalso take up glucose from the blood for conversion into triglycerides. This occurs in the same way as in the liver, except that these tissues do not release the triglycerides thus produced as VLDL into the blood. Adipose tissue cells store the triglycerides in their fat droplets, ultimately to release them again as free fatty acids and glycerol into the blood (as describedabove), when the plasma concentration of insulin is low, and that of glucagon and/or epinephrine is high.[33]Mammary glands discharge the fat (as cream fat droplets) into the milk that they produce under the influence of theanterior pituitaryhormoneprolactin.
All cells in the body need to manufacture and maintain their membranes and the membranes of their organelles. Whether they rely entirely on free fatty acids absorbed from the blood, or are able to synthesize their own fatty acids from blood glucose, is not known. The cells of thecentral nervous systemwill almost certainly have the capability of manufacturing their own fatty acids, as these molecules cannot reach them through theblood–brain barrier.[34]However, it is unknown how they are reached by theessential fatty acids,which mammals cannot synthesize themselves but are nevertheless important components of cell membranes (andother functionsdescribed above).
Fatty acid synthesis[edit]

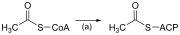
Much likebeta-oxidation,straight-chain fatty acid synthesis occurs via the six recurring reactions shown below, until the 16-carbonpalmitic acidis produced.[35][36]
The diagrams presented show how fatty acids are synthesized in microorganisms and list the enzymes found inEscherichia coli.[35]These reactions are performed byfatty acid synthaseII (FASII), which in general contains multiple enzymes that act as one complex. FASII is present inprokaryotes,plants, fungi, and parasites, as well as inmitochondria.[37]
In animals as well as some fungi such as yeast, these same reactions occur on fatty acid synthase I (FASI), a large dimeric protein that has all of the enzymatic activities required to create a fatty acid. FASI is less efficient than FASII; however, it allows for the formation of more molecules, including "medium-chain" fatty acids via early chain termination.[37]Enzymes, acyltransferases and transacylases, incorporate fatty acids in phospholipids, triacylglycerols, etc. by transferring fatty acids between an acyl acceptor and donor. They also have the task of synthesizing bioactive lipids as well as their precursor molecules.[38]
Once a 16:0 carbon fatty acid has been formed, it can undergo a number of modifications, resulting in desaturation and/or elongation. Elongation, starting with stearate (18:0), is performed mainly in theendoplasmic reticulumby several membrane-bound enzymes. The enzymatic steps involved in the elongation process are principally the same as those carried out byfatty acid synthesis,but the four principal successive steps of the elongation are performed by individual proteins, which may be physically associated.[39][40]
| Step | Enzyme | Reaction | Description |
|---|---|---|---|
| (a) | Acetyl-CoA:ACP transacylase | Activates acetyl-CoA for reaction with malonyl-ACP | |
| (b) | Malonyl-CoA:ACP transacylase | Activates malonyl-CoA for reaction with acetyl-ACP | |
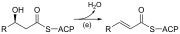
| (c) | 3-ketoacyl-ACP synthase | 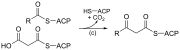 |
Reacts ACP-bound acyl chain with chain-extending malonyl-ACP |
| (d) | 3-ketoacyl-ACP reductase | Reduces the carbon 3 ketone to a hydroxyl group | |
| (e) | 3-Hydroxyacyl ACP dehydrase | Eliminates water | |
| (f) | Enoyl-ACP reductase | Reduces the C2-C3 double bond. |
Abbreviations: ACP –Acyl carrier protein,CoA –Coenzyme A,NADP –Nicotinamide adenine dinucleotide phosphate.
Note that during fatty synthesis the reducing agent isNADPH,whereasNADis the oxidizing agent inbeta-oxidation(the breakdown of fatty acids to acetyl-CoA). This difference exemplifies a general principle that NADPH is consumed during biosynthetic reactions, whereas NADH is generated in energy-yielding reactions.[34](Thus NADPH is also required for the synthesis ofcholesterolfrom acetyl-CoA; while NADH is generated duringglycolysis.) The source of the NADPH is two-fold. Whenmalateis oxidatively decarboxylated by “NADP+-linked malic enzyme "pyruvate,CO2and NADPH are formed. NADPH is also formed by thepentose phosphate pathwaywhich converts glucose into ribose, which can be used in synthesis ofnucleotidesandnucleic acids,or it can be catabolized to pyruvate.[34]
Glycolytic end products are used in the conversion of carbohydrates into fatty acids[edit]
In humans, fatty acids are formed from carbohydrates predominantly in the liver andadipose tissue,as well as in themammary glandsduring lactation. Thepyruvateproduced byglycolysisis an important intermediary in the conversion of carbohydrates into fatty acids and cholesterol.[34]This occurs via the conversion of pyruvate into acetyl-CoA in the mitochondrion. However, this acetyl-CoA needs to be transported into cytosol where the synthesis of fatty acids and cholesterol occurs. This cannot occur directly. To obtain cytosolic acetyl-CoA,citrate(produced by the condensation of acetyl-CoA with oxaloacetate) is removed from thecitric acid cycleand carried across the inner mitochondrial membrane into the cytosol.[34]There it is cleaved byATP citrate lyaseinto acetyl-CoA and oxaloacetate. The oxaloacetate is returned to mitochondrion as malate (and then converted back into oxaloacetate to transfer more acetyl-CoA out of the mitochondrion).[41]The cytosolic acetyl-CoA is carboxylated byacetyl-CoA carboxylaseintomalonyl-CoA,the first committed step in the synthesis of fatty acids.[41][42]
Regulation of fatty acid synthesis[edit]
Acetyl-CoA is formed intomalonyl-CoAbyacetyl-CoA carboxylase,at which point malonyl-CoA is destined to feed into the fatty acid synthesis pathway. Acetyl-CoA carboxylase is the point of regulation in saturated straight-chain fatty acid synthesis, and is subject to bothphosphorylationandallosteric regulation.Regulation by phosphorylation occurs mostly in mammals, while allosteric regulation occurs in most organisms. Allosteric control occurs as feedback inhibition by palmitoyl-CoA and activation by citrate. When there are high levels of palmitoyl-CoA, the final product of saturated fatty acid synthesis, it allosterically inactivates acetyl-CoA carboxylase to prevent a build-up of fatty acids in cells. Citrate acts to activate acetyl-CoA carboxylase under high levels, because high levels indicate that there is enough acetyl-CoA to feed into theKrebs cycleand produce energy.[43]
High plasma levels ofinsulinin the blood plasma (e.g. after meals) cause the dephosphorylation and activation of acetyl-CoA carboxylase, thus promoting the formation of malonyl-CoA from acetyl-CoA, and consequently the conversion of carbohydrates into fatty acids, whileepinephrineandglucagon(released into the blood during starvation and exercise) cause the phosphorylation of this enzyme, inhibitinglipogenesisin favor of fatty acid oxidation viabeta-oxidation.[34][42]
Disorders[edit]
Disorders of fatty acid metabolism can be described in terms of, for example,hypertriglyceridemia(too high level oftriglycerides), or other types ofhyperlipidemia.These may be familial or acquired.
Familial types of disorders of fatty acid metabolism are generally classified asinborn errors of lipid metabolism.These disorders may be described asfatty acid oxidation disordersor as alipid storage disorders,and are any one of severalinborn errors of metabolismthat result from enzyme or transport protein defects affecting the ability of the body tooxidizefatty acidsin order to produce energy within muscles, liver, and othercelltypes. When a fatty acid oxidation disorder affects the muscles, it is ametabolic myopathy.
Moreover, cancer cells can display irregular fatty acid metabolism with regard to bothfatty acid synthesis[44]and mitochondrialfatty acid oxidation(FAO)[45]that are involved in diverse aspects of tumorigenesis and cell growth.
References[edit]
- ^abcdefStryer, Lubert (1995). "Fatty acid metabolism.".In: Biochemistry(Fourth ed.). New York: W.H. Freeman and Company. pp. 603–628.ISBN0-7167-2009-4.
- ^abcdOxidation of fatty acids
- ^Zechner R, Strauss JG, Haemmerle G, Lass A, Zimmermann R (2005). "Lipolysis: pathway under construction".Curr. Opin. Lipidol.16(3): 333–40.doi:10.1097/01.mol.0000169354.20395.1c.PMID15891395.S2CID35349649.
- ^Mobilization and cellular uptake of stored fats (triacylglycerols) (with animation)
- ^Stahl, Andreas (1 February 2004). "A current review of fatty acid transport proteins (SLC27)".Pflügers Archiv: European Journal of Physiology.447(5): 722–727.doi:10.1007/s00424-003-1106-z.PMID12856180.S2CID2769738.
- ^Anderson, Courtney M.; Stahl, Andreas (April 2013)."SLC27 fatty acid transport proteins".Molecular Aspects of Medicine.34(2–3): 516–528.doi:10.1016/j.mam.2012.07.010.PMC3602789.PMID23506886.
- ^Ebert, D.; Haller, RG.; Walton, ME. (Jul 2003)."Energy contribution of octanoate to intact rat brain metabolism measured by13C nuclear magnetic resonance spectroscopy ".J Neurosci.23(13): 5928–35.doi:10.1523/JNEUROSCI.23-13-05928.2003.PMC6741266.PMID12843297.
- ^Marin-Valencia, I.; Good, LB.; Ma, Q.; Malloy, CR.; Pascual, JM. (Feb 2013)."Heptanoate as a neural fuel: energetic and neurotransmitter precursors in normal and glucose transporter I-deficient (G1D) brain".J Cereb Blood Flow Metab.33(2): 175–82.doi:10.1038/jcbfm.2012.151.PMC3564188.PMID23072752.
- ^Stryer, Lubert (1995). "Fatty acid metabolism.".In: Biochemistry(Fourth ed.). New York: W.H. Freeman and Company. pp. 770–771.ISBN0-7167-2009-4.
- ^abcdefghiStryer, Lubert (1995).Biochemistry(Fourth ed.). New York: W.H. Freeman and Company. pp. 510–515, 581–613, 775–778.ISBN0-7167-2009-4.
- ^Activation and transportation of fatty acids to the mitochondria via the carnitine shuttle (with animation)
- ^Vivo, Darryl C.; Bohan, Timothy P.; Coulter, David L.; Dreifuss, Fritz E.; Greenwood, Robert S.; Nordli, Douglas R.; Shields, W. Donald; Stafstrom, Carl E.; Tein, Ingrid (1998)."l-Carnitine Supplementation in Childhood Epilepsy: Current Perspectives".Epilepsia.39(11): 1216–1225.doi:10.1111/j.1528-1157.1998.tb01315.x.ISSN0013-9580.PMID9821988.S2CID28692799.
- ^Oxidation of odd carbon chain length fatty acids
- ^Oxidation of unsaturated fatty acids
- ^Wongkittichote P, Ah Mew N, Chapman KA (December 2017)."Propionyl-CoA carboxylase - A review".Molecular Genetics and Metabolism.122(4): 145–152.doi:10.1016/j.ymgme.2017.10.002.PMC5725275.PMID29033250.
- ^Halarnkar PP, Blomquist GJ (1989). "Comparative aspects of propionate metabolism".Comp. Biochem. Physiol. B.92(2): 227–31.doi:10.1016/0305-0491(89)90270-8.PMID2647392.
- ^Stryer, Lubert (1995).Biochemistry(Fourth ed.). New York: W.H. Freeman and Company. p. 777.ISBN0-7167-2009-4.
- ^Sloan, A.W; Koeslag, J.H.; Bredell, G.A.G. (1973). "Body composition work capacity and work efficiency of active and inactive young men".European Journal of Applied Physiology.32:17–24.doi:10.1007/bf00422426.S2CID39812342.
- ^Ruddick JA (1972). "Toxicology, metabolism, and biochemistry of 1,2-propanediol".Toxicol Appl Pharmacol.21(1): 102–111.doi:10.1016/0041-008X(72)90032-4.PMID4553872.
- ^abcGlew, Robert H."You Can Get There From Here: Acetone, Anionic Ketones and Even-Carbon Fatty Acids can Provide Substrates for Gluconeogenesis".Nigerian Journal of Physiological Science.25(1). Invited review: 2–4. Archived fromthe originalon 26 September 2013.Retrieved7 August2016.
- ^abcPark, Sung M.; Klapa, Maria I.; Sinskey, Anthony J.; Stephanopoulos, Gregory (1999)."Metabolite and isotopomer balancing in the analysis of metabolic cycles: II. Applications"(PDF).Biotechnology and Bioengineering.62(4): 398.doi:10.1002/(sici)1097-0290(19990220)62:4<392::aid-bit2>3.0.co;2-s.ISSN0006-3592.PMID9921151.
- ^Miller DN, Bazzano G; Bazzano (1965). "Propanediol metabolism and its relation to lactic acid metabolism".Ann NY Acad Sci.119(3): 957–973.Bibcode:1965NYASA.119..957M.doi:10.1111/j.1749-6632.1965.tb47455.x.PMID4285478.S2CID37769342.
- ^D. L. Vander Jagt; B. Robinson; K. K. Taylor; L. A. Hunsaker (1992)."Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic complications".The Journal of Biological Chemistry.267(7): 4364–4369.doi:10.1016/S0021-9258(18)42844-X.PMID1537826.
- ^Stryer, Lubert (1995). "Signal transduction cascades.".In: Biochemistry(Fourth ed.). New York: W.H. Freeman and Company. pp. 343–350.ISBN0-7167-2009-4.
- ^abStryer, Lubert (1995). "Eicosanoid hormones are derived from fatty acids.".In: Biochemistry(Fourth ed.). New York: W.H. Freeman and Company. pp. 624–627.ISBN0-7167-2009-4.
- ^Nelson, Randy F. (2005).An introduction to behavioral endocrinology(3rd ed.). Sunderland, Mass: Sinauer Associates. p. 100.ISBN978-0-87893-617-5.
- ^abDigestion of fats (triacylglycerols)
- ^Hofmann AF (1963)."The function of bile salts in fat absorption. The solvent properties of dilute micellar solutions of conjugated bile salts".Biochem. J.89(1): 57–68.doi:10.1042/bj0890057.PMC1202272.PMID14097367.
- ^Stryer, Lubert (1995). "Membrane structures and dynamics.".In: Biochemistry(Fourth ed.). New York: W.H. Freeman and Company. pp. 268–270.ISBN0-7167-2009-4.
- ^Gropper, Sareen S.; Smith, Jack L. (2013).Advanced nutrition and human metabolism(6th ed.). Belmont, CA: Wadsworth/Cengage Learning.ISBN978-1133104056.
- ^Williams, Peter L.; Warwick, Roger; Dyson, Mary; Bannister, Lawrence H. (1989). "Angiology.".In: Gray's Anatomy(Thirty-seventh ed.). Edinburgh: Churchill Livingstone. pp. 841–843.ISBN0443-041776.
- ^Stryer, Lubert (1995). "Biosynthesis of membrane lipids and steroids.".In: Biochemistry(Fourth ed.). New York: W.H. Freeman and Company. pp. 697–700.ISBN0-7167-2009-4.
- ^Stralfors, Peter; Honnor, Rupert C. (1989)."Insulin-induced dephosphorylation of hormone-sensitive lipase".European Journal of Biochemistry.182(2): 379–385.doi:10.1111/j.1432-1033.1989.tb14842.x.PMID2661229.
- ^abcdefStryer, Lubert (1995).Biochemistry(Fourth ed.). New York: W.H. Freeman and Company. pp. 559–565, 614–623.ISBN0-7167-2009-4.
- ^abDijkstra, Albert J., R. J. Hamilton, and Wolf Hamm. "Fatty Acid Biosynthesis." Trans Fatty Acids. Oxford: Blackwell Pub., 2008. 12. Print.
- ^"MetaCyc pathway: superpathway of fatty acids biosynthesis".MetaCyc Metabolic Pathway Database.BioCyc. (E. coli).
- ^abChristie, William W. (20 April 2011). "Fatty Acids: Straight-chain Saturated, Structure, Occurrence and Biosynthesis". InAmerican Oil Chemists' Society(ed.).AOCS Lipid Library.Archived fromthe originalon 2011-07-21.Retrieved2011-05-02.
- ^Yamashita, Atsushi; Hayashi, Yasuhiro; Nemoto-Sasaki, Yoko; Ito, Makoto; Oka, Saori; Tanikawa, Takashi; Waku, Keizo; Sugiura, Takayuki (2014-01-01). "Acyltransferases and transacylases that determine the fatty acid composition of glycerolipids and the metabolism of bioactive lipid mediators in mammalian cells and model organisms".Progress in Lipid Research.53:18–81.doi:10.1016/j.plipres.2013.10.001.ISSN0163-7827.PMID24125941.
- ^"MetaCyc pathway: stearate biosynthesis I (animals)".MetaCyc Metabolic Pathway Database.BioCyc.
- ^"MetaCyc pathway: very long chain fatty acid biosynthesis II".MetaCyc Metabolic Pathway Database.BioCyc.
- ^abFerre, P.; F. Foufelle (2007)."SREBP-1c Transcription Factor and Lipid Homeostasis: Clinical Perspective".Hormone Research.68(2): 72–82.doi:10.1159/000100426.PMID17344645.Retrieved2010-08-30.
this process is outlined graphically in page 73
- ^abVoet, Donald; Judith G. Voet; Charlotte W. Pratt (2006).Fundamentals of Biochemistry, 2nd Edition.John Wiley and Sons, Inc. pp.547, 556.ISBN978-0-471-21495-3.
- ^Diwan, Joyce J. "Fatty Acid Synthesis." Rensselaer Polytechnic Institute (RPI):: Architecture, Business, Engineering, IT, Humanities, Science. Web. 30 Apr. 2011. <"Fatty Acid Synthesis".Archived fromthe originalon 2011-06-07.Retrieved2011-05-02.>.
- ^Ezzeddini R, Taghikhani M, Somi MH, Samadi N, Rasaee, MJ (May 2019)."Clinical importance of FASN in relation to HIF-1α and SREBP-1c in gastric adenocarcinoma".Life Sciences.224:169–176.doi:10.1016/j.lfs.2019.03.056.PMID30914315.S2CID85532042.
- ^Ezzeddini R, Taghikhani M, Salek Farrokhi A, Somi MH, Samadi N, Esfahani A, Rasaee, MJ (May 2021)."Downregulation of fatty acid oxidation by involvement of HIF-1α and PPARγ in human gastric adenocarcinoma and its related clinical significance".Journal of Physiology and Biochemistry.77(2): 249–260.doi:10.1007/s13105-021-00791-3.ISSN1138-7548.PMID33730333.S2CID232300877.