Ferric

Inchemistry,iron(III)orferricrefers to theelementironin its +3oxidation state.Ferric chlorideis an alternative name foriron(III) chloride(FeCl3). The adjectiveferrousis used instead foriron(II)salts, containing the cation Fe2+.The wordferricis derived from theLatinwordferrum,meaning "iron".
Although often abbreviated asFe3+,that naked ion does not exist except under extreme conditions. Iron(III) centres are found in many compounds andcoordination complexes,where Fe(III) is bonded to several ligands. A molecular ferric complex is theanionferrioxalate,[Fe(C2O4)3]3−,with threebidentateoxalateions surrounding the Fe core. Relative to lower oxidation states, ferric is less common inorganoiron chemistry,but theferroceniumcation[Fe(C2H5)2]+is well known.
Iron(III) in biology
[edit]All known forms of life require iron, which usually exists in Fe(II) or Fe(III) oxidation states.[1]Manyproteinsin living beings contain iron(III) centers. Examples of suchmetalloproteinsincludeoxyhemoglobin,ferredoxin,and thecytochromes.Many organisms, from bacteria to humans, store iron as microscopic crystals (3 to 8 nm in diameter) ofiron(III) oxide hydroxide,inside a shell of the proteinferritin,from which it can be recovered as needed.[2]
Insufficient iron in the human diet causesanemia.Animals and humans can obtain the necessary iron from foods that contain it in assimilable form, such as meat. Other organisms must obtain their iron from the environment. However, iron tends to form highly insoluble iron(III) oxides/hydroxides in aerobic (oxygenated) environment, especially incalcareous soils.Bacteriaandgrassescan thrive in such environments by secreting compounds calledsiderophoresthat form soluble complexes with iron(III), that can be reabsorbed into the cell. (The other plants instead encourage the growth around their roots of certain bacteria thatreduceiron(III) to the more soluble iron(II).)[3]
The insolubility of iron(III) compounds is also responsible for the low levels of iron in seawater, which is often the limiting factor for the growth of the microscopic plants (phytoplankton) that are the basis of the marine food web.[4]
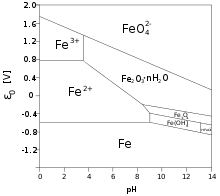
Iron(III) salts and complexes
[edit]Typically iron(III) salts, like the "chloride"areaquo complexeswith the formulas[Fe(H2O)5Cl]2+,[Fe(H2O)4Cl2]+,and [Fe(H2O)3Cl3].Iron(III) nitrate dissolved in water to give[Fe(H2O)6]3+ions. In these complexes, the protons are acidic. Eventually these solutionshydrolyzeproducingiron(III) hydroxideFe(OH)3that further converts to polymeric oxide-hydroxide via the process calledolation.These hydroxidesprecipitatesout of the solution as solids. That reaction liberateshydrogenionsH+lowering the pH of its solutions. Theequilibriaare elaborate:[5]
- [Fe(H2O)6]3+⇌ [Fe(H2O)5OH]2++ H+
- [Fe(H2O)5OH]2+⇌ [Fe(H2O)4(OH)2]++ H+
- 2 [Fe(H2O)4(OH)2]+⇌ [Fe2(H2O)8(OH)2]+2+ 2 H2O
Variouschelatingcompounds prevent the polymerization. These same ligands can even dissolve iron(III) oxides and hydroxides. One of these ligands isEDTA,which is often used to dissolve iron deposits or added to fertilizers to make iron in the soil available (soluble) to plants.Citratealso solubilizes ferric ion at neutral pH, although its complexes are less stable than those of EDTA. Many chelating ligands - thesiderophores- are produced naturally to dissolve iron(III) oxides.
The aquo ligands on iron(III) complexes are labile. This behavior is visualized by the color change brought about by reaction with thiocyanate:
- [Fe(H2O)6]3++ SCN−⇌ [Fe(SCN)(H2O)5]2++ H2O
Whereas[Fe(H2O)6]3+is nearly colorless,[Fe(SCN)(H2O)5]2+is deep red.
While iron(III) aquo complexes tend to convert to polymeric oxy-hydroxides, iron(III) complexes with other ligands form stable solutions. The complex with1,10-phenanthrolinebipyridineis soluble and can sustain reduction to it iron(II) derivative:

Iron(III) minerals and other solids
[edit]
Iron(III) is found in many minerals and solids, e.g.,oxideFe2O3(hematite) andiron(III) oxide-hydroxideFeO(OH)are extremely insoluble reflecting theirpolymericstructure.Rustis a mixture of iron(III) oxide and oxide-hydroxide that usually forms when iron metal is exposed tohumidair. Unlike thepassivatingoxide layers that are formed by other metals, likechromiumandaluminum,rust flakes off, because it is bulkier than the metal that formed it. Therefore, unprotected iron objects will in time be completely turned into rust.
Bonding
[edit]
Iron(III) is a d5center, meaning that the metal has five "valence" electrons in the 3d orbital shell. The number and type of ligands bound to iron(III) determine how these electrons arrange themselves. With so-called "strong field ligands" such ascyanide,the five electrons pair up as best they can. Thusferricyanide([Fe(CN)6]3−has only one unpaired electron. It is low-spin. With so-called "weak field ligands" such aswater,the five electrons are unpaired. Thusaquo complex([Fe(H2O)6]3+has only five unpaired electrons. It is high-spin. With chloride, iron(III) forms tetrahedral complexes, e.g. ([Fe(Cl)4]−.Tetrahedral complexes are high spin. The magnetism of ferric complexes can show when they are high or low spin.
See also
[edit]- Ferric chloride– Inorganic compound(Iron(III) chloride)
- Ferric oxide– Chemical compound(Iron(III) oxide)
- Ferric fluoride– chemical compound(Iron(III) fluoride)
- Ferrous– The element iron in its +2 oxidation state
References
[edit]- ^"Iron integral to the development of life on Earth – and the possibility of life on other planets".University of Oxford.7 December 2021.Retrieved9 May2022.
- ^Berg, Jeremy Mark; Lippard, Stephen J. (1994).Principles of bioinorganic chemistry.Sausalito, Calif: University Science Books.ISBN0-935702-73-3.
- ^H. Marschner and V. Römheld (1994): "Strategies of plants for acquisition of iron".Plant and Soil,volume 165, issue 2, pages 261–274.doi:10.1007/BF00008069
- ^Boyd PW, Watson AJ, Law CS, et al. (October 2000). "A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization".Nature.407(6805): 695–702.Bibcode:2000Natur.407..695B.doi:10.1038/35037500.PMID11048709.S2CID4368261.
- ^Earnshaw, A.; Greenwood, N. N. (1997).Chemistry of the elements(2nd ed.). Oxford: Butterworth-Heinemann.ISBN0-7506-3365-4.
