Transition metal oxo complex

Atransition metal oxo complexis acoordination complexcontaining anoxo ligand.Formally O2–,an oxo ligand can be bound to one or more metal centers, i.e. it can exist as a terminal or (most commonly) asbridging ligands.Oxo ligands stabilize high oxidation states of a metal.[1]They are also found in severalmetalloproteins,for example inmolybdenum cofactorsand in many iron-containing enzymes. One of the earliest synthetic compounds to incorporate an oxo ligand ispotassium ferrate(K2FeO4), which was likely prepared byGeorg E. Stahlin 1702.[2]
Reactivity
[edit]Olation and acid-base reactions
[edit]
A common reaction exhibited by metal-oxo compounds isolation,the condensation process that converts low molecular weight oxides to polymers with M-O-M linkages. Olation often begins with the deprotonation of a metal-hydroxo complex. It is the basis for mineralization and the precipitation of metal oxides. For the oxides of d0 metals, VV,NbV,TaV,MoVI,and WVI,the olation process affordspolyoxometallates,a large class of molecular metal oxides.
Oxygen-atom transfer
[edit]Metal oxo complexes are intermediates in manymetal-catalyzed oxidation reactions.Oxygen-atom transfer is common reaction of particular interest inorganic chemistryandbiochemistry.[3]Some metal-oxos are capable of transferring their oxo ligand to organic substrates. One such example of this type of reactivity is from the enzyme superfamilymolybdenum oxotransferase.
Inwater oxidation catalysis,metal oxo complexes are intermediates in the conversion of water to O2.
Hydrogen-atom abstraction
[edit]Transition metal-oxo's are also capable of abstracting strong C–H, N–H, and O–H bonds.Cytochrome P450contains a high-valent iron-oxo which is capable of abstracting hydrogen atoms from strong C–H bonds.[4]
Molecular oxides
[edit]Some of the longest known and most widely used oxo compounds are oxidizing agents such aspotassium permanganate(KMnO4) andosmium tetroxide(OsO4).[5]Compounds such as these are widely used for converting alkenes tovicinaldiolsand alcohols to ketones or carboxylic acids.[1]More selective or gentler oxidizing reagents includepyridinium chlorochromate(PCC) andpyridinium dichromate(PDC).[1]Metal oxo species are capable of catalytic, including asymmetric oxidations of various types. Some metal-oxo complexes promoteC-H bond activation,converting hydrocarbons to alcohols.[6]

Selection of molecular metal oxides. From left,vanadyl chloride(d0), a tungsten oxo carbonyl (d2),permanganate(d0), [ReO2(pyridine)4]+(d2), simplified view of compound I (a state ofcytochrome P450,d4), and Ir(O)(mesityl)3(d4).[7]
Metalloenzymes
[edit]Iron(IV)-oxo species
[edit]
Iron(IV)-oxo compounds are intermediates in many biological oxidations:
- Alpha-ketoglutarate-dependent hydroxylasesactivate O2by oxidative decarboxylation ofketoglutarate,generating Fe(IV)=O centers, i.e.ferryl,that hydroxylate a variety of hydrocarbon substrates.[9]
- Cytochrome P450enzymes, use ahemecofactor,insert ferryl oxygen into saturated C–H bonds,[10]epoxidize olefins,[11][12]and oxidize aromatic groups.[13]
- Methane monooxygenase(MMO) oxidizes methane to methanol via oxygen atom transfer from an iron-oxo intermediate at its non-heme diiron center.[14]Much effort is aimed at reproducing reactions with synthetic catalysts.[6]
Molybdenum/tungsten oxo species
[edit]
The oxo ligand (or analogous sulfido ligand) is nearly ubiquitous in molybdenum and tungsten chemistry, appearing in the ores containing these elements, throughout their synthetic chemistry, and also in their biological role (aside from nitrogenase). The biologically transported species and starting point for biosynthesis is generally accepted to be oxometallates MoO42−or WO42−.All Mo/W enzymes, again exceptnitrogenase,are bound to one or moremolybdopterinprosthetic group. The Mo/W centers generally cycle between hexavalent (M(VI)) and tetravalent (M(IV)) states. Although there is some variation among these enzymes, members from all three families involve oxygen atom transfer between the Mo/W center and the substrate.[15]Representative reactions from each of the three structural classes are:
- Sulfite oxidase:SO32−+ H2O → SO42−+ 2 H++ 2 e−
- DMSO reductase:H3C–S(O)–CH3(DMSO) + 2 H++ 2 e−→ H3C–S–CH3(DMS) + H2O
- Aldehyde ferredoxin oxidoreductase:R–CHO + H2O → R–CO2H + 2 H++ 2 e−
The three different classes of molybdenum cofactors are shown in the adjacent figure. The biological use of tungsten mirrors that of molybdenum.[16]
Oxygen-evolving complex
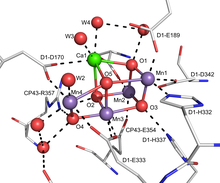
[edit]The active site for theoxygen-evolving complex(OEC) ofphotosystem II(PSII) is a Mn4O5Ca centre with several bridging oxo ligands that participate in the oxidation of water to molecular oxygen.[17]The OEC is proposed to utilize a terminal oxo intermediate as a part of the water oxidation reaction. This complex is responsible for the production of nearly all of earth's molecular oxygen. This key link in theoxygen cycleis necessary for much of thebiodiversitypresent on earth.
The "oxo wall"
[edit]
The term "oxo wall" is a theory used to describe the fact that no terminal oxo complexes are known for metal centers with octahedral symmetry and d-electron counts beyond 5.[18][19]
Oxo compounds for the vanadium through iron triads (Groups3-8) are well known, whereas terminal oxo compounds for metals in the cobalt through zinc triads (Groups 9-12) are rare and invariably feature metals with coordination numbers lower than 6. This trend holds for other metal-ligand multiple bonds. Claimed exceptions to this rule[20][21][22]have been retracted.[23][24][25]
The iridium oxo complex Ir(O)(mesityl)3may appear to be an exception to the oxo-wall rule, but it is not because the complex is non-octahedral.[7]The trigonal symmetry reorders the metal d-orbitals below the degenerate MO π* pair. In three-fold symmetric complexes, multiple MO bonding is allowed for as many as 7 d-electrons.[18]
Terminal oxo ligands are also rather rare for the titanium triad, especially zirconium and hafnium and are unknown for group 3 metals (scandium, yttrium, and lanthanum).[1]
See also
[edit]References
[edit]- ^abcdNugent, W. A., Mayer, J. M. "Metal-Ligand Multiple Bonds." John Wiley & Sons, New York, 1988.
- ^Delaude, Lionel; Laszlo, Pierre (1996-01-01)."A Novel Oxidizing Reagent Based on Potassium Ferrate(VI)1".The Journal of Organic Chemistry.61(18): 6360–6370.doi:10.1021/jo960633p.ISSN0022-3263.PMID11667478.
- ^Holm, R. H. (1987). "Metal-centered oxygen atom transfer reactions".Chem. Rev.87(6): 1401–1449.doi:10.1021/cr00082a005.
- ^Meunier, Bernard; de Visser, Samuël P.; Shaik, Sason (2004). "Mechanism of Oxidation Reactions Catalyzed by Cytochrome P450 Enzymes".Chemical Reviews.104(9): 3947–3980.doi:10.1021/cr020443g.ISSN0009-2665.PMID15352783.
- ^Du, G.; Abu-Omar, M. M. (2008). "Oxo and Imido Complexes of Rhenium and Molybdenum in Catalytic Reductions".Current Organic Chemistry.12(14): 1185–1198.doi:10.2174/138527208785740238.
- ^abGunay, A.; Theopold, K. H. (2010). "C-H Bond Activations by Metal Oxo Compounds".Chem. Rev.110(2): 1060–1081.doi:10.1021/cr900269x.PMID20143877.
- ^abHay-Motherwell, Robyn S.;Wilkinson, Geoffrey;Hussain-Bates, Bilquis; Hursthouse, Michael B. (1993). "Synthesis and X-ray Crystal Structure of Oxotrimesityl-Iridium(V)".Polyhedron.12(16): 2009–2012.doi:10.1016/S0277-5387(00)81474-6.
- ^Huang, Xiongyi; Groves, John T. (2017)."Beyond Ferryl‑Mediated Hydroxylation: 40 Years of the rebound mechanism and C–H activation".J Biol Inorg Chem.22(2–3): 185–207.doi:10.1007/s00775-016-1414-3.PMC5350257.PMID27909920.
- ^Hausinger, R. P. (January–February 2004). "Fe(II)/α-Ketoglutarate-Dependent Hydroxylases and Related Enzymes".Crit. Rev. Biochem. Mol. Biol.39(1): 21–68.doi:10.1080/10409230490440541.PMID15121720.S2CID85784668.
- ^Ortiz de Montellano, Paul R. (2010)."Hydrocarbon Hydroxylation by Cytochrome P450 Enzymes".Chemical Reviews.110(2): 932–948.doi:10.1021/cr9002193.ISSN0009-2665.PMC2820140.PMID19769330.
- ^Coon, M. J. (1998-01-20)."Epoxidation of olefins by cytochrome P450: Evidence from site-specific mutagenesis for hydroperoxo-iron as an electrophilic oxidant".Proceedings of the National Academy of Sciences.95(7): 3555–60.Bibcode:1998PNAS...95.3555V.doi:10.1073/pnas.95.7.3555.PMC19874.PMID9520404.
- ^Farinas, Edgardo T; Alcalde, Miguel; Arnold, Frances (2004). "Alkene epoxidation catalyzed by cytochrome P450 BM-3 139-3".Tetrahedron.60(3): 525–528.doi:10.1016/j.tet.2003.10.099.ISSN0040-4020.
- ^Korzekwa, Kenneth; Trager, William;Gouterman, Martin;Spangler, Dale; Loew, Gilda (1985). "Cytochrome P450 mediated aromatic oxidation: a theoretical study".Journal of the American Chemical Society.107(14): 4273–4279.doi:10.1021/ja00300a033.ISSN0002-7863.
- ^Brunold, T.C. (2007)."Synthetic Iron-Oxo 'Diamond Core' Mimics Structure of Key IIntermediate in Methane Monooxygenase Catalytic Cycle".Proc. Natl. Acad. Sci. U.S.A.104(52): 20641–20642.Bibcode:2007PNAS..10420641B.doi:10.1073/pnas.0710734105.PMC2409203.PMID18093936.
- ^Schwarz, G.; Mendel, R. R.; Ribbe, M. W. (2009). "Molybdenum Cofactors, Enzymes and Pathways".Nature.460(7257): 839–847.Bibcode:2009Natur.460..839S.doi:10.1038/nature08302.PMID19675644.S2CID205217953.
- ^Mukund, S.; Adams, M. W. W. (1996)."Molybdenum and Vanadium Do not Replace Tungsten in the Catalytically Active Forms of the Three Tungstoenzymes in the Hyperthermophilic Archaeon Pyrococcus furiosus".J. Bacteriol.178(1): 163–167.doi:10.1128/jb.178.1.163-167.1996.PMC177634.PMID8550411.
- ^abUmena, Yasufumi; Kawakami, Keisuke; Shen, Jian-Ren; Kamiya, Nobuo (2011)."Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å"(PDF).Nature.473(7345): 55–60.Bibcode:2011Natur.473...55U.doi:10.1038/nature09913.ISSN0028-0836.PMID21499260.S2CID205224374.
- ^abcWinkler, Jay R.;Gray, Harry B.(2012). "Electronic Structures of Oxo-Metal Ions". InMingos, David Michael P.;Day, Peter;Dahl, Jens Peder (eds.).Molecular Electronic Structures of Transition Metal Complexes I.Structure and Bonding. Vol. 142.Springer Nature.pp. 17–28.doi:10.1007/430_2011_55.ISBN978-3-642-27369-8.
- ^Larson, Virginia A.; Battistella, Beatrice; Ray, Kallol; Lehnert, Nicolai; Nam, Wonwoo (2020). "Iron and manganese oxo complexes, oxo wall and beyond".Nature Reviews Chemistry.4(8): 404–419.doi:10.1038/s41570-020-0197-9.S2CID220295993.
- ^Anderson, Travis M.; Neiwert, Wade A.; Kirk, Martin L.; Piccoli, Paula M. B.; Schultz, Arthur J.; Koetzle, Thomas F.; Musaev, Djamaladdin G.; Morokuma, Keiji; Cao, Rui; Hill, Craig L. (2004-12-17)."A Late-Transition Metal Oxo Complex: K7Na9[O=PtIV(H2O)L2], L = [PW9O34]9-".Science.306(5704): 2074–2077.doi:10.1126/science.1104696.ISSN0036-8075.PMID15564312.S2CID41123922.(Retracted, seedoi:10.1126/science.337.6092.290-a,PMID22822129,Retraction Watch)
- ^Anderson, Travis M.; Cao, Rui; Slonkina, Elena; Hedman, Britt; Hodgson, Keith O.; Hardcastle, Kenneth I.; Neiwert, Wade A.; Wu, Shaoxiong; Kirk, Martin L.; Knottenbelt, Sushilla; Depperman, Ezra C. (2005-08-01)."A Palladium-Oxo Complex. Stabilization of This Proposed Catalytic Intermediate by an Encapsulating Polytungstate Ligand".Journal of the American Chemical Society.127(34): 11948–11949.doi:10.1021/ja054131h.ISSN0002-7863.PMID16117527.(Retracted, seedoi:10.1021/ja207910h,PMID22873777,Retraction Watch)
- ^Cao, Rui; Anderson, Travis M.; Piccoli, Paula M. B.; Schultz, Arthur J.; Koetzle, Thomas F.; Geletii, Yurii V.; Slonkina, Elena; Hedman, Britt; Hodgson, Keith O.; Hardcastle, Kenneth I.; Fang, Xikui (2007-09-01)."Terminal Gold-Oxo Complexes".Journal of the American Chemical Society.129(36): 11118–11133.doi:10.1021/ja072456n.ISSN0002-7863.PMID17711276.(Retracted, seedoi:10.1021/ja207909y,PMID22873776,Retraction Watch)
- ^O’Halloran, Kevin P.; Zhao, Chongchao; Ando, Nicole S.; Schultz, Arthur J.; Koetzle, Thomas F.; Piccoli, Paula M. B.; Hedman, Britt;Hodgson, Keith O.;et al. (2012). "Revisiting the Polyoxometalate-Based Late-Transition-Metal-Oxo Complexes: The" Oxo Wall "Stands".Inorganic Chemistry.51(13): 7025–7031.doi:10.1021/ic2008914.PMID22694272.
- ^Ritter, Stephen K. (June 12, 2012)."Metal-Oxo Papers Retracted".cen.acs.org.Retrieved2021-05-15.
- ^Hadlington2012-06-14T00:00:00+01:00, Simon."Oxo wall still stands as inorganic papers retracted".Chemistry World.Retrieved2021-05-15.
{{cite web}}:CS1 maint: numeric names: authors list (link)
