Quinolone antibiotic
| Quinolone | |
|---|---|
| Drug class | |
 The second generation fluoroquinolone, ciprofloxacin. The two ringed nitrogen containing system with a ketone is called aquinolone. | |
| Class identifiers | |
| Use | Bacterial infection |
| ATC code | J01M |
| Clinical data | |
| Drugs.com | Drug Classes |
| External links | |
| MeSH | D015363 |
| Legal status | |
| In Wikidata | |
Quinolone antibioticsconstitute a large group ofbroad-spectrumbacteriocidalsthat share abicyclic core structurerelated to the substance4-quinolone.[1]They are used in human and veterinary medicine to treat bacterialinfections,as well as in animal husbandry, specifically poultry production.[2]
Nearly all quinolone antibiotics in use arefluoroquinolones,which contain afluorineatom in their chemical structure and are effective against bothGram-negativeandGram-positivebacteria. One example isciprofloxacin,one of the most widely used antibiotics worldwide.[3][4]
Medical uses
[edit]

Fluoroquinolones are often used for genitourinary infections[5]and are widely used in the treatment of hospital-acquired infections associated with urinary catheters. In community-acquired infections, they are recommended only when risk factors for multidrug resistance are present or after other antibiotic regimens have failed. However, for serious acute cases ofpyelonephritisor bacterialprostatitiswhere the person may need to be hospitalised, fluoroquinolones are recommended as first-line therapy.[6]
Due to people withsickle-cell diseasebeing at increased risk for developingosteomyelitisfrom theSalmonella,fluoroquinolones are the "drugs of choice" due to their ability to enter bone tissue without chelating it, astetracyclinesare known to do.[citation needed]
Fluoroquinolones are featured prominently in guidelines for the treatment of hospital-acquired pneumonia.[7]
Children
[edit]In most countries, fluoroquinolones are approved for use in children only under narrowly defined circumstances, owing in part to the observation of high rates of musculoskeletal adverse events in fluoroquinolone-treated juvenile animals. In the UK, the prescribing indications for fluoroquinolones for children are severely restricted. Only inhalantanthraxandpseudomonalinfections incystic fibrosisinfections are licensed indications in the UK due to ongoing safety concerns. In a study comparing the safety and efficacy of levofloxacin to that ofazithromycinorceftriaxonein 712 children with community-acquired pneumonia, serious adverse events were experienced by 6% of those treated with levofloxacin and 4% of those treated with comparator antibiotics. Most of these were considered by the treating physician to be unrelated or doubtfully related to the study drug. Two deaths were observed in the levofloxacin group, neither of which was thought to be treatment-related. Spontaneous reports to the U.S. FDA Adverse Effects Reporting System at the time of the 20 September 2011 U.S. FDA Pediatric Drugs Advisory Committee included musculoskeletal events (39, including five cases of tendon rupture) andcentral nervous systemevents (19, including five cases of seizures) as the most common spontaneous reports between April 2005 and March 2008. An estimated 130,000 pediatric prescriptions for levofloxacin were filled on behalf of 112,000 pediatric patients during that period.[8]
Meta-analyses conclude that fluoroquinolones pose little or no additional risk to children compared to other antibiotic classes.[9][10][11]Fluoroquinolone use in children may be appropriate when the infection is caused bymultidrug-resistant bacteria,or when alternative treatment options require parenteral administration and oral therapy is preferred.[12]
Adverse effects
[edit]While typical drug side effects reactions are mild to moderate, sometimes serious adverse effects occur.
Boxed warnings
[edit]In 2008, the U.S. FDA addedblack box warningson all fluoroquinolones, advising of the increased risk of tendon damage.[13]In 2016, the FDA found that systemic use (by mouth or injection) of fluoroquinolones was associated with "disabling and potentially permanent serious side effects" involving the tendons, muscles, joints, nerves, and central nervous system, concluding that these side effects generally outweigh the benefits for people with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections when other treatment options are available.[14]Concerns regardinglow blood sugarandmental health problemswere added in 2018.[15]
Tendons
[edit]Quinolones are associated with a small risk of tendonitis and tendon rupture; a 2013 review found the incidence of tendon injury among those taking fluoroquinolones to be between 0.08 and 0.20%.[16]The risk appears to be higher among people older than 60 and those also taking corticosteroids;[16]the risk also may be higher among people who are male, have a pre-existing joint or tendon issue, have kidney disease, or are highly active.[17]Some experts have advised avoidance of fluoroquinolones in athletes.[17]If tendonitis occurs, it generally appears within one month, and the most common tendon injured appears to be theAchilles tendon.[16]The cause is not well understood.[16]
Nervous system
[edit]Nervous-system effects include insomnia, restlessness, and rarely, seizure, convulsions, and psychosis.[18]Other rare and serious adverse events have been observed with varying degrees of evidence for causation.[19][20][21][22]
Aortic dissection
[edit]Fluoroquinolones can increase the rate of rare but serious tears in the aorta by 31% compared to other antibiotics.[23]People at increased risk include those with aortic aneurysm, hypertension, certain genetic conditions such asMarfan syndromeandEhlers-Danlos syndrome,and the elderly. For these people, fluoroquinolones should be used only when no other treatment options are available.[24]
Colitis
[edit]Clostridium difficilecolitismay occur in connection with the use of any antibacterial drug, especially those with a broad spectrum of activity such as clindamycin, cephalosporins, and fluoroquinolones. Fluoroquinoline treatment is associated with risk that is similar to[25]or less than[26][27]that associated with broad spectrum cephalosporins. Fluoroquinoline administration may be associated with the acquisition and outgrowth of a particularly virulentClostridiumstrain.[28]
Other
[edit]More generally, fluoroquinolones are tolerated, with typical drug side effects being mild to moderate.[29]Common side effects include gastrointestinal effects such as nausea, vomiting, and diarrhea, as well as headache and insomnia. Postmarketing surveillance has revealed a variety of relatively rare but serious adverse effects associated with all members of the fluoroquinolone antibacterial class. Among these, tendon problems and exacerbation of the symptoms of the neurological disordermyasthenia gravisare the subject of"black box" warningsin the United States.[30][31]
A 2018 EU-wide review of fluoroquinolones concluded that they are associated with serious side effects including tendonitis, tendon rupture, arthralgia, pain in extremities, gait disturbance, neuropathies associated with paraesthesia, depression, fatigue, memory impairment, sleep disorders, and impaired hearing, vision, taste and smell. Tendon damage (especially to Achilles tendon but also other tendons) can occur within 48 hours of starting fluoroquinolone treatment but the damage may be delayed several months after stopping treatment.[32]
The overall rate of adverse events in people treated with fluoroquinolones is roughly similar to that seen in people treated with other antibiotic classes.[26][33][34][35]A U.S. Centers for Disease Control and Prevention study found people treated with fluoroquinolones experienced adverse events severe enough to lead to an emergency department visit more frequently than those treated withcephalosporinsormacrolides,but less frequently than those treated withpenicillins,clindamycin,sulfonamides,orvancomycin.[36]
Fluoroquinolones prolong the heart'sQT intervalby blocking voltage-gated potassium channels.[37]Prolongation of the QT interval can lead totorsades de pointes,a life-threateningarrhythmia,but in practice, this appears relatively uncommon in part because the most widely prescribed fluoroquinolones (ciprofloxacin and levofloxacin) only minimally prolong the QT interval.[38]
In 2019 study byJournal of the American College of Cardiologyit was discovered that fluoroquinolones could increase the risk for heart valve diseases.[39]
Events that may occur in acute overdose are rare, and includekidney failureand seizure.[40]Susceptible groups of patients, such as children and the elderly, are at greater risk of adverse reactions during therapeutic use.[29][41][42]
Mechanism of toxicity
[edit]The mechanisms of the toxicity of fluoroquinolones have been attributed to their interactions with different receptor complexes, such as blockade of the GABAAreceptor complex within the central nervous system, leading to excitotoxic type effects[31]and oxidative stress.[43]
Interactions
[edit]Products containing multivalentcations,such as aluminium- or magnesium-containingantacids,and products containing calcium, iron, or zinc invariably result in marked reduction of oral absorption of fluoroquinolones.[44]Other drugs that interact with fluoroquinolones includesucralfate,probenecid,cimetidine,theophylline,warfarin,antiviral agents,phenytoin,cyclosporine,rifampin,pyrazinamide,andcycloserine.[44]
Administration of quinolone antibiotics to abenzodiazepine-dependentindividual can precipitate acutebenzodiazepine withdrawalsymptoms due to quinolones displacing benzodiazepines from their binding sites.[45]Fluoroquinolones have varying specificity forcytochrome P450,so may have interactions with drugs cleared by those enzymes; the order from most P450-inhibitory to least, is enoxacin > ciprofloxacin > norfloxacin > ofloxacin, levofloxacin, trovafloxacin, gatifloxacin, moxifloxacin.[44]
Contraindications
[edit]Quinolones are not recommended in people withepilepsy,Marfan's syndrome,Ehlers-Danlos Syndrome,[46]QT prolongation,pre-existing CNS lesions, or CNS inflammation, or who have had astroke.[31]They are best avoided in the athlete population.[47]Safety concerns exist for fluoroquinolone use during pregnancy, so they are contraindicated unless no other safe alternative antibiotic exists.[48]However, one meta-analysis looking at the outcome of pregnancies involving quinolone use in the first trimester found no increased risk of malformations.[49]They are also contraindicated in children due to the risks of damage to the musculoskeletal system.[50]Their use in children is not absolutely contraindicated, however. For certain severe infections where other antibiotics are not an option, their use can be justified.[51]Quinolones should also not be given to people with a knownhypersensitivityto the drug class.[52][53]
The basicpharmacophore,or active structure, of the fluoroquinolone class is based upon thequinolinering system.[54]The addition of thefluorineatomat C6 distinguishes the successive-generation fluoroquinolones from the first-generation of quinolones. The addition of the C6 fluorine atom has since been demonstrated not to be required for theantibacterialactivity of this class (circa1997).[55]
Antibiotic misuse and bacterial resistances
[edit]Because the use of broad-spectrum antibiotics encourages the spread of multidrug-resistant strains and the development ofClostridium difficileinfections, treatment guidelines often recommend minimizing the use of fluoroquinolones and other broad-spectrum antibiotics in less severe infections and in those in which risk factors for multidrug resistance are not present. It has been recommended that fluoroquinolones not be used as a first-line agent for community-acquired pneumonia,[56]instead recommendingmacrolideor doxycycline as first-line agents. The Drug-ResistantStreptococcus pneumoniaeWorking Group recommends fluoroquinolones be used for the ambulatory treatment of community-acquired pneumonia only after other antibiotic classes have been tried and failed, or in cases with demonstrated drug-resistantStreptococcus pneumoniae.[57]
Resistanceto quinolones can evolve rapidly, even during a course of treatment. Numerouspathogens,includingEscherichia coli,commonly exhibit resistance.[58]Widespread veterinary usage of quinolones, in particular in Europe, has been implicated.[59]
Fluoroquinolones had become the class of antibiotics most commonly prescribed to adults in 2002. Nearly half (42%) of these prescriptions were for conditions not approved by the U.S. FDA, such asacute bronchitis,otitis media,and acute upper respiratory tract infection, according to a study supported in part by theAgency for Healthcare Research and Quality.[60][61]In addition, they are commonly prescribed for medical conditions, such as acute respiratory illness, that are usually caused by viral infections.[62]
Three mechanisms of resistance are known.[63]Some types ofeffluxpumps can act to decrease intracellular quinolone concentration.[64]In gram-negative bacteria, plasmid-mediated resistance genes produce proteins that can bind toDNA gyrase,protecting it from the action of quinolones. Finally,mutationsat key sites inDNA gyraseortopoisomerase IVcan decrease their binding affinity to quinolones, decreasing the drugs' effectiveness.[citation needed]
Mechanism of action
[edit]
Quinolones are chemotherapeutic bactericidal drugs. They interfere withDNA replicationby preventing bacterial DNA from unwinding and duplicating.[65]Specifically, they inhibit the ligase activity of thetype II topoisomerases,DNA gyrase and topoisomerase IV, which cut DNA to introduce supercoiling, while leaving nuclease activity unaffected. With the ligase activity disrupted, these enzymes release DNA with single- and double-strand breaks that lead to cell death.[66]The majority of quinolones in clinical use are fluoroquinolones, which have afluorineatomattached to the central ring system, typically at the6-position or C-8 position.Most of them are named with the-oxacinsuffix. First and second generation quinolones are largely active against Gram-negative bacteria, whereas third and fourth generation quinolones have increased activity against Gram-positive and anaerobic bacteria.[67]Some quinolones containing aromatic substituents at their C-7 positions are highly active against eukaryotic type II topoisomerase.[68]
It has also been proposed that quinolone antibiotics cause oxidation of guanine nucleotides in the bacterial nucleotide pool, and that this process contributes to the cytotoxicity of these agents.[69]The incorporation of oxidized guanine nucleotides intoDNAcould be bactericidal. Bacterial cytotoxicity could arise from incomplete repair of closely spaced8-oxo-2'-deoxyguanosinein the DNA resulting in double-strand breaks.[69]
Cellular uptake
[edit]Fluoroquinolones can enter in cells easily viaporins,so are often used to treatintracellularpathogenssuch asLegionella pneumophilaandMycoplasma pneumoniae.For many Gram-negative bacteria, DNA gyrase is the target, whereas topoisomerase IV is the target for many Gram-positive bacteria.[citation needed]
Eukaryotic cells are not believed to contain DNA gyrase or topoisomerase IV. However, debate exists concerning whether the quinolones still have such an adverse effect on the DNA of healthy cells. Some compounds in this class have been shown to inhibit the synthesis ofmitochondrial DNA.[70][71][72][73]
Pharmacology
[edit]The basicpharmacophore,or active structure, of the fluoroquinolone class is based upon the quinoline ring system.[74]Various substitutions made to the quinoline ring resulted in the development of numerous fluoroquinolone drugs. The addition of thefluorineatomat C-6 distinguishes the successive-generation fluoroquinolones from the first-generation quinolones, although examples are known that omit the atom while retaining antibacterial activity.[55]
Pharmacokinetics
[edit]| Drug | Dosagea (mg) |
BA(%) | Cmax (μg/mL) |
tmax (h) |
AUC (μg • h/mL) |
t1/2 (h) |
Vd/F (L/kg) |
Protein binding(%) |
Excreted unchanged (%) |
Dose adjustment | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Renal | Hepatic | ||||||||||
| Ciprofloxacin | 500 750 |
70 70 |
2.30 3.00 |
1.2 1.2 |
10.1 14.0 |
3.5 3.5 |
3.5 3.5 |
30 30 |
34 34 |
Yes Yes |
No No |
| Garenoxacin | 400 600 |
ND 92 |
5.0 10.4 |
ND 1.2 |
60 96.7 |
14.2 9.8 |
ND ND |
75 ND |
40 ND |
ND ND |
ND ND |
| Gatifloxacin | 400 | 96 | 3.86 | 1.5 | 33.8 | 8.0 | 1.8 | 20 | 76 | Yes | No |
| Gemifloxacin | 320 640 |
70 70 |
1.19 2.29 |
1.2 1.2 |
7.3 15.9 |
8.0 8.0 |
3.5 3.5 |
60 60 |
27 27 |
Yes Yes |
No No |
| Levofloxacin | 500 750 |
99 99 |
5.08 7.13 |
1.7 1.7 |
48.0 82.0 |
6.9 6.9 |
1.1 1.1 |
31 31 |
83 83 |
Yes Yes |
ND ND |
| Moxifloxacin | 200 400 |
86 86 |
1.16 3.34 |
1.7 1.7 |
15.4 33.8 |
12.1 12.1 |
3.3 3.3 |
47 47 |
19 19 |
No No |
No No |
| a= Dosage applies only to CmaxandAUC.The other parameters an average of the values available in the literature irrespective of dosage. | |||||||||||
History
[edit]
Although not formally a quinolone,nalidixic acidis considered the first quinolone drug. It was introduced in 1962 for treatment ofurinary tract infections(UTIs) in humans.[76]Nalidixic acid was discovered by George Lesher and coworkers in adistillateduring an attempt atchloroquinesynthesis.[77]Nalidixic acid is thus considered to be the predecessor of all members of the quinolone family, including the second, third and fourth generations commonly known as fluoroquinolones. Since the introduction of nalidixic acid, more than 10,000analogshave been synthesized, but only a handful have found their way into clinical practice. The first generation also included other quinolone drugs, such aspipemidic acid,oxolinic acid,andcinoxacin,which were introduced in the 1970s. They proved to be only marginal improvements over nalidixic acid.[78]
These drugs were widely used as a first-line treatment for many infections, including very commons ones such as acute sinusitis, acute bronchitis, and uncomplicated UTIs.[79]Reports of serious adverse events began emerging, and the FDA first added a black-box warning to fluoroquinolones in July 2008 for the increased risk of tendinitis and tendon rupture. In February 2011, the risk of worsening symptoms for those with myasthenia gravis was added to the warning. In August 2013, the agency required updates to the labels to describe the potential for irreversible peripheral neuropathy (serious nerve damage).[citation needed]
In November 2015, an FDA Advisory Committee discussed the risks and benefits of fluoroquinolones for the treatment of acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated UTIs based on new safety information. The new information focused on two or more side effects occurring at the same time and causing the potential for irreversible impairment. The advisory committee concluded that the serious risks associated with the use of fluoroquinolones for these types of uncomplicated infections generally outweighed the benefits for patients with other treatment options.[79][80][81][82][83]The 21-member joint committee overwhelmingly recommended stronger label warnings on the containers because of rare but sometimes devastating side effects.[84]
On 12 May 2016, the FDA issued a drug safety communication advising that fluoroquinolones should be reserved for these conditions only when no other options are available due to potentially permanent, disabling side effects occurring together. The drug safety communication also announced the required labeling updates to reflect this new safety information.[79]The FDA put out another label change in July 2017, strengthening the warnings about potentially disabling adverse effects and limiting use of these drugs to second-line treatments for acute sinusitis, acute bronchitis, and uncomplicated UTIs.[79]
Generations
[edit]The first generation of the quinolones began following introduction of the related, but structurally distinct naphthyridine-family nalidixic acid in 1962 for treatment of UTIs in humans.[85]Nalidixic acid was discovered by George Lesher and coworkers in a chemicaldistillateduring an attempt at synthesis of the chloroquinoline antimalarial agent,chloroquine.[86]Naphthyridone and quinolone classes of antibiotics prevent bacterial DNA replication by inhibition of DNA unwinding events, and can be both bacteriostatic and bacteriocidal.[65](SeeMechanism of Actionlater.) The majority of quinolones in clinical use belong to the second generation class of "fluoroquinolones", which have a true quinoline framework, maintain the C-3 carboxylic acid group, and add afluorineatomto the all-carbon containing ring, typically at the C-6 or C-8 positions.[67]
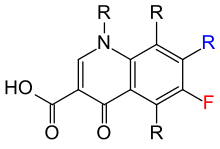
Quinolones can be classified into generations based on their antibacterial spectrums.[87][88]The earlier-generation agents are, in general, more narrow-spectrum than the later ones, but no standard is employed to determine which drug belongs to which generation. The only universal standard applied is the grouping of the non-fluorinateddrugs found within this class (quinolones) within the first-generation heading. As such, a wide variation exists within the literature dependent upon the methods employed by the authors.[citation needed]
The first generation is rarely used. Frequently prescribed drugs aremoxifloxacin,ciprofloxacin,levofloxacin.
First generation
[edit]- flumequine(veterinary use)
- oxolinic acid
- rosoxacin
Structurally related first-generation drugs, but formally not 4-quinolones, includecinoxacin,[89]nalidixic acid,[89]andpiromidic acid,pipemidic acid
Second generation
[edit]The second-generation class is sometimes subdivided into "Class 1" and "Class 2".[89]
- ciprofloxacin[89][90]
- fleroxacin
- lomefloxacin[89]
- nadifloxacin
- norfloxacin
- ofloxacin[89]
- pefloxacin
- rufloxacin
A structurally related second-generation drug, but formally not a 4-quinolone, isenoxacin.[89]
Third generation
[edit]Unlike the first and second generations, the third generation is active againststreptococci.[89]
A structurally related third-generation drug, but formally not a 4-quinolone, istosufloxacin(Ozex, Tosacin).
Fourth generation
[edit]Fourth-generation fluoroquinolones act at DNA gyrase and topoisomerase IV.[92]This dual action slows development of resistance.[dubious–discuss]
- clinafloxacin[90]
- gatifloxacin[93]
- moxifloxacin[89]
- sitafloxacin
- prulifloxacin
- besifloxacin
- delafloxacin
Two structurally related third-generation drugs, but formally not 4-quinolones, aregemifloxacinandtrovafloxacin(removed from clinical use).[89][90]
In development:
Veterinary use
[edit]Quinolones have been widely used inanimal husbandry,and several agents have veterinary-specific applications.
- danofloxacin– 2nd gen, related to ciprofloxacin
- difloxacin– 2nd/3rd gen, related to temafloxacin
- enrofloxacin– 2nd gen, metabolizes into ciprofloxacin
- ibafloxacin– 3rd gen, related to levofloxacin
- marbofloxacin– 3rd gen, related to levofloxacin
- orbifloxacin– 3rd gen, related to sparfloxacin
- sarafloxacin– 2nd/3rd gen, related to difloxacin
References
[edit]![]() This article incorporatespublic domain materialfromFDA updates warnings for fluoroquinolone antibiotics.United States Department of Health and Human Services.
This article incorporatespublic domain materialfromFDA updates warnings for fluoroquinolone antibiotics.United States Department of Health and Human Services.
- ^Andriole VT (1989).The Quinolones.Academic Press.
- ^Johnson JR, Murray AC, Gajewski A, Sullivan M, Snippes P, Kuskowski MA, Smith KE (July 2003)."Isolation and molecular characterization of nalidixic acid-resistant extraintestinal pathogenic Escherichia coli from retail chicken products".Antimicrobial Agents and Chemotherapy.47(7): 2161–2168.doi:10.1128/AAC.47.7.2161-2168.2003.PMC161843.PMID12821463.
- ^Andersson MI, MacGowan AP (May 2003)."Development of the quinolones".The Journal of Antimicrobial Chemotherapy.51(Suppl S1): 1–11.doi:10.1093/jac/dkg212.PMID12702698.
- ^Heeb S, Fletcher MP, Chhabra SR, Diggle SP, Williams P, Cámara M (March 2011)."Quinolones: from antibiotics to autoinducers".FEMS Microbiology Reviews.35(2): 247–274.doi:10.1111/j.1574-6976.2010.00247.x.PMC3053476.PMID20738404.
- ^Balch J, Schoen JH, Patel PK (1 October 2017)."Should Physicians Consider the Environmental Effects of Prescribing Antibiotics?".AMA Journal of Ethics.19(10): 957–965.doi:10.1001/journalofethics.2017.19.10.peer1-1710.ISSN2376-6980.
- ^Liu H, Mulholland SG (July 2005). "Appropriate antibiotic treatment of genitourinary infections in hospitalized patients".The American Journal of Medicine.118(Suppl 7A): 14S–20S.doi:10.1016/j.amjmed.2005.05.009.PMID15993673.
- ^American Thoracic Society, Infectious Diseases Society of America (February 2005). "Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia".American Journal of Respiratory and Critical Care Medicine.171(4): 388–416.doi:10.1164/rccm.200405-644ST.PMID15699079.S2CID14907563.
- ^"Adverse Event Review: Levaquin® (levofloxacin): Pediatric Advisory Committee Meeting"(PDF).Food and Drug Administration. 18 November 2008.
- ^Sung L, Manji A, Beyene J, Dupuis LL, Alexander S, Phillips R, Lehrnbecher T (May 2012). "Fluoroquinolones in children with fever and neutropenia: a systematic review of prospective trials".The Pediatric Infectious Disease Journal.31(5): 431–435.doi:10.1097/INF.0b013e318245ab48.PMID22189521.S2CID52835801.
- ^Rosanova MT, Lede R, Capurro H, Petrungaro V, Copertari P (December 2010). "[Assessing fluoroquinolones as risk factor for musculoskeletal disorders in children: a systematic review and meta-analysis]".Archivos Argentinos de Pediatria(in Spanish).108(6): 524–531.doi:10.1590/S0325-00752010000600008(inactive 31 January 2024).PMID21132249.
{{cite journal}}:CS1 maint: DOI inactive as of January 2024 (link) - ^Forsythe CT, Ernst ME (November 2007)."Do fluoroquinolones commonly cause arthropathy in children?".CJEM.9(6): 459–462.doi:10.1017/s1481803500015517.PMID18072993.
- ^Bradley JS, Jackson MA (October 2011)."The use of systemic and topical fluoroquinolones".Pediatrics.128(4): e1034–e1045.doi:10.1542/peds.2011-1496.PMID21949152.
- ^"FDA orders 'black box' label on some antibiotics".CNN. 8 July 2008.Retrieved8 July2008.
- ^"FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together".FDA.12 May 2016.
- ^"Safety Alerts for Human Medical Products – Fluoroquinolone Antibiotics: FDA Requires Labeling Changes Due to Low Blood Sugar Levels and Mental Health Side Effects".fda.gov.Retrieved13 July2018.
- ^abcdStephenson AL, Wu W, Cortes D, Rochon PA (September 2013). "Tendon Injury and Fluoroquinolone Use: A Systematic Review".Drug Safety.36(9): 709–721.doi:10.1007/s40264-013-0089-8.PMID23888427.S2CID24948660.
- ^abLewis T, Cook J (2014)."Fluoroquinolones and tendinopathy: a guide for athletes and sports clinicians and a systematic review of the literature".Journal of Athletic Training.49(3): 422–427.doi:10.4085/1062-6050-49.2.09.PMC4080593.PMID24762232.
- ^Galatti L, Giustini SE, Sessa A, Polimeni G, Salvo F, Spina E, Caputi AP (March 2005). "Neuropsychiatric reactions to drugs: an analysis of spontaneous reports from general practitioners in Italy".Pharmacological Research.51(3): 211–216.doi:10.1016/j.phrs.2004.08.003.PMID15661570.
- ^Babar SM (October 2013). "SIADH associated with ciprofloxacin".The Annals of Pharmacotherapy.47(10): 1359–1363.doi:10.1177/1060028013502457.PMID24259701.S2CID36759747.
- ^Rouveix B (November–December 2006). "[Clinically significant toxicity and tolerance of the main antibiotics used in lower respiratory tract infections]".Médecine et Maladies Infectieuses.36(11–12): 697–705.doi:10.1016/j.medmal.2006.05.012.PMID16876974.
- ^Mehlhorn AJ, Brown DA (November 2007). "Safety concerns with fluoroquinolones".The Annals of Pharmacotherapy.41(11): 1859–1866.doi:10.1345/aph.1K347.PMID17911203.S2CID26411679.
- ^Jones SE, Smith RH (March 1997)."Quinolones may induce hepatitis".The BMJ.314(7084): 869.doi:10.1136/bmj.314.7084.869.PMC2126221.PMID9093098.
- ^Newton ER, Akerman AW, Strassle PD, Kibbe MR (March 2021)."Association of Fluoroquinolone Use With Short-term Risk of Development of Aortic Aneurysm".JAMA Surgery.156(3): 264–272.doi:10.1001/jamasurg.2020.6165.PMC7788511.PMID33404647.
- ^"FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients".FDA.20 December 2018.Retrieved9 February2019.
- ^Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston DD, Sferra TJ, et al. (September 2013)."Community-associated Clostridium difficile infection and antibiotics: a meta-analysis".The Journal of Antimicrobial Chemotherapy.68(9): 1951–1961.doi:10.1093/jac/dkt129.PMID23620467.
- ^abLevine JG, Szarfman A (15 December 2006)."Data Mining Analysis of Multiple Antibiotics in AERS"(Microsoft PowerPoint).U.S. Food and Drug Administration.
- ^Slimings C, Riley TV (April 2014)."Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis".The Journal of Antimicrobial Chemotherapy.69(4): 881–891.doi:10.1093/jac/dkt477.PMID24324224.
- ^Vardakas KZ, Konstantelias AA, Loizidis G, Rafailidis PI, Falagas ME (November 2012)."Risk factors for development of Clostridium difficile infection due to BI/NAP1/027 strain: a meta-analysis".International Journal of Infectious Diseases.16(11): e768–e773.doi:10.1016/j.ijid.2012.07.010.PMID22921930.
- ^abOwens RC, Ambrose PG (July 2005)."Antimicrobial safety: focus on fluoroquinolones".Clinical Infectious Diseases.41(Suppl 2): S144–S157.doi:10.1086/428055.PMID15942881.
- ^"Information for Healthcare Professionals: Fluoroquinolone Antimicrobial Drugs [ciprofloxacin (Marketed as Cipro and generic ciprofloxacin), ciprofloxacin extended-release (Marketed as Cipro XR and Proquin XR), gemifloxacin (Marketed as Factive), levofloxacin (Marketed as Levaquin), moxifloxacin (Marketed as Avelox), norfloxacin (Marketed as Noroxin), and ofloxacin (Marketed as Floxin)]".Food and Drug Administration.Archived fromthe originalon 30 October 2017.Retrieved16 December2019.
- ^abcDe Sarro A, De Sarro G (March 2001). "Adverse reactions to fluoroquinolones. an overview on mechanistic aspects".Current Medicinal Chemistry.8(4): 371–384.doi:10.2174/0929867013373435.PMID11172695.
- ^"Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics".European Medicines Agency.11 March 2019.
- ^Skalsky K, Yahav D, Lador A, Eliakim-Raz N, Leibovici L, Paul M (April 2013)."Macrolides vs. quinolones for community-acquired pneumonia: meta-analysis of randomized controlled trials".Clinical Microbiology and Infection.19(4): 370–378.doi:10.1111/j.1469-0691.2012.03838.x.PMID22489673.
- ^Falagas ME, Matthaiou DK, Vardakas KZ (December 2006). "Fluoroquinolones vs beta-lactams for empirical treatment of immunocompetent patients with skin and soft tissue infections: a meta-analysis of randomized controlled trials".Mayo Clinic Proceedings.81(12): 1553–1566.doi:10.4065/81.12.1553.PMID17165634.
- ^Van Bambeke F, Tulkens PM (2009). "Safety profile of the respiratory fluoroquinolone moxifloxacin: comparison with other fluoroquinolones and other antibacterial classes".Drug Safety.32(5): 359–378.doi:10.2165/00002018-200932050-00001.PMID19419232.S2CID19026852.
- ^Shehab N, Patel PR, Srinivasan A, Budnitz DS (September 2008)."Emergency department visits for antibiotic-associated adverse events".Clinical Infectious Diseases.47(6): 735–743.doi:10.1086/591126.PMID18694344.
- ^Heidelbaugh JJ, Holmstrom H (April 2013). "The perils of prescribing fluoroquinolones".The Journal of Family Practice.62(4): 191–197.PMID23570031.
- ^Rubinstein E, Camm J (April 2002)."Cardiotoxicity of fluoroquinolones".The Journal of Antimicrobial Chemotherapy.49(4): 593–596.doi:10.1093/jac/49.4.593.PMID11909831.
- ^Bakalar N (17 September 2019)."Antibiotics Tied to Heart Valve Problems".The New York Times.ISSN0362-4331.Retrieved24 August2021.
- ^Nelson LH, Flomenbaum N, Goldfrank LR, Hoffman RL, Howland MD, Lewin NA (2006).Goldfrank's toxicologic emergencies.New York: McGraw-Hill, Medical Pub. Division.ISBN978-0-07-143763-9.
- ^Iannini PB (June 2007). "The safety profile of moxifloxacin and other fluoroquinolones in special patient populations".Current Medical Research and Opinion.23(6): 1403–1413.doi:10.1185/030079907X188099.PMID17559736.S2CID34091286.
- ^Farinas ER, et al. (Public Health Service Food and Drug Administration Center for Drug Evaluation and Research) (1 March 2005)."Consult: One-Year Post Pediatric Exclusivity Postmarketing Adverse Events Review"(PDF).Food and Drug Administration.Retrieved31 August2009.
- ^Saint F, Salomon L, Cicco A, de la Taille A, Chopin D, Abbou CC (December 2001). "[Tendinopathy associated with fluoroquinolones: individuals at risk, incriminated physiopathologic mechanisms, therapeutic management]".Progres en Urologie(in French).11(6): 1331–1334.PMID11859676.
- ^abcFish DN (October 2001). "Fluoroquinolone adverse effects and drug interactions".Pharmacotherapy.21(10 Pt 2): 253S–272S.doi:10.1592/phco.21.16.253S.33993.PMID11642691.S2CID29617455.
- ^Ford C, Law F (July 2014)."Guidance for the use and reduction of misuse of benzodiazepines and other hypnotics and anxiolytics in general practice"(PDF).SMMGP. Archived fromthe original(PDF)on 6 July 2017.Retrieved24 July2017.
- ^"Fluoroquinolones Antibiotic Alert – especially with EDS".29 August 2013.
- ^2011 Documentlevaquinadversesideeffect.comArchived17 June 2021 at theWayback Machine
- ^Nardiello S, Pizzella T, Ariviello R (March 2002). "[Risks of antibacterial agents in pregnancy]".Le Infezioni in Medicina.10(1): 8–15.PMID12700435.
- ^Bar-Oz B, Moretti ME, Boskovic R, O'Brien L, Koren G (April 2009). "The safety of quinolones--a meta-analysis of pregnancy outcomes".European Journal of Obstetrics, Gynecology, and Reproductive Biology.143(2): 75–78.doi:10.1016/j.ejogrb.2008.12.007.PMID19181435.
- ^Noel GJ, Bradley JS, Kauffman RE, Duffy CM, Gerbino PG, Arguedas A, et al. (October 2007). "Comparative safety profile of levofloxacin in 2523 children with a focus on four specific musculoskeletal disorders".The Pediatric Infectious Disease Journal.26(10): 879–891.doi:10.1097/INF.0b013e3180cbd382.PMID17901792.S2CID26457648.
- ^Leibovitz E (February 2006). "The use of fluoroquinolones in children".Current Opinion in Pediatrics.18(1): 64–70.doi:10.1097/01.mop.0000192520.48411.fa.PMID16470165.S2CID37437573.
- ^Janssen Pharmaceutica(September 2008)."Highlights of Prescribing Information"(PDF).Food and Drug Administration.
- ^Scherer K, Bircher AJ (January 2005). "Hypersensitivity reactions to fluoroquinolones".Current Allergy and Asthma Reports.5(1): 15–21.doi:10.1007/s11882-005-0049-1.PMID15659258.S2CID31447696.
- ^Schaumann R, Rodloff AC (January 2007)."Activities of Quinolones Against Obligately Anaerobic Bacteria"(PDF).Anti-Infective Agents in Medicinal Chemistry.6(1): 49–56.doi:10.2174/187152107779314179.Archived fromthe original(PDF)on 16 June 2010.Retrieved6 May2011.
- ^abHong CY, Kim SH, Kim YK (22 July 1997). "Novel 5-amino-6-methylquinolone antibacterials: A new class of non-6-fluoroquinolones".Bioorganic & Medicinal Chemistry Letters.7(14): 1875–1878.doi:10.1016/S0960-894X(97)00324-7.
- ^Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. (March 2007)."Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults".Clinical Infectious Diseases.44(Suppl 2): S27–S72.doi:10.1086/511159.PMC7107997.PMID17278083.
- ^MacDougall C, Guglielmo BJ, Maselli J, Gonzales R (March 2005)."Antimicrobial drug prescribing for pneumonia in ambulatory care".Emerging Infectious Diseases.11(3): 380–384.doi:10.3201/eid1103.040819.PMC3298265.PMID15757551.
- ^Jacobs M (2005). "Worldwide overview of antimicrobial Resistance.".International Symposium on Antimicrobial Agents and Resistance. Drugs.pp. 542–546.
- ^Nelson JM, Chiller TM, Powers JH, Angulo FJ (April 2007)."Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story".Clinical Infectious Diseases.44(7): 977–980.doi:10.1086/512369.PMID17342653.
- ^Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS (March 2005). "Fluoroquinolone prescribing in the United States: 1995 to 2002".The American Journal of Medicine.118(3): 259–268.doi:10.1016/j.amjmed.2004.09.015.PMID15745724.
- ^K08 HS14563 and HS11313
- ^Neuhauser MM, Weinstein RA, Rydman R, Danziger LH, Karam G, Quinn JP (February 2003)."Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use".JAMA.289(7): 885–888.doi:10.1001/jama.289.7.885.PMID12588273.
From 1995 to 2002, inappropriate antibiotic prescribing for acute respiratory infections, which are usually caused by viruses and thus are not responsive to antibiotics, declined from 61 to 49 percent. However, the use of broad-spectrum antibiotics such as the fluoroquinolones, jumped from 41 to 77 percent from 1995 to 2001. Overuse of these antibiotics will eventually render them useless for treating antibiotic-resistant infections, for which broad-spectrum antibiotics are supposed to be reserved.
- ^Robicsek A, Jacoby GA, Hooper DC (October 2006). "The worldwide emergence of plasmid-mediated quinolone resistance".The Lancet. Infectious Diseases.6(10): 629–640.doi:10.1016/S1473-3099(06)70599-0.PMID17008172.
- ^Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T (July 1998)."NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli".Antimicrobial Agents and Chemotherapy.42(7): 1778–1782.doi:10.1128/AAC.42.7.1778.PMC105682.PMID9661020.
- ^abHooper DC (March–April 2001)."Emerging mechanisms of fluoroquinolone resistance".Emerging Infectious Diseases.7(2): 337–341.doi:10.3201/eid0702.010239.PMC2631735.PMID11294736.
- ^Aldred KJ, Kerns RJ, Osheroff N (March 2014)."Mechanism of quinolone action and resistance".Biochemistry.53(10): 1565–1574.doi:10.1021/bi5000564.PMC3985860.PMID24576155.
- ^abAndriole VT (July 2005)."The quinolones: past, present, and future".Clinical Infectious Diseases.41(S2): S113–S119.doi:10.1086/428051.PMID15942877.
- ^Elsea SH, Osheroff N, Nitiss JL (July 1992)."Cytotoxicity of quinolones toward eukaryotic cells. Identification of topoisomerase II as the primary cellular target for the quinolone CP-115,953 in yeast".The Journal of Biological Chemistry.267(19): 13150–13153.doi:10.1016/S0021-9258(18)42185-0.PMID1320012.
- ^abFoti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC (April 2012)."Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics".Science.336(6079). New York, N.Y.: 315–9.Bibcode:2012Sci...336..315F.doi:10.1126/science.1219192.PMC3357493.PMID22517853.
- ^Bergan T (1988). "Pharmacokinetics of fluorinated quinolones".The quinolones.Academic Press. pp. 119–154.
- ^Bergan T, Dalhoff A, Thorsteinsson SB (July 1985). "A review of the pharmacokinetics and tissue penetration of ciprofloxacin.".Ciprofloxacin A new 4–quinolone.Hong Kong: Sieber and McIntyre. pp. 23–36.
- ^Castora FJ, Vissering FF, Simpson MV (September 1983). "The effect of bacterial DNA gyrase inhibitors on DNA synthesis in mammalian mitochondria".Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression.740(4): 417–427.doi:10.1016/0167-4781(83)90090-8.PMID6309236.
- ^Suto MJ, Domagala JM, Roland GE, Mailloux GB, Cohen MA (December 1992). "Fluoroquinolones: relationships between structural variations, mammalian cell cytotoxicity, and antimicrobial activity".Journal of Medicinal Chemistry.35(25): 4745–4750.doi:10.1021/jm00103a013.PMID1469702.
- ^Schaumann R, Rodloff AC (January 2007)."Activities of Quinolones Against Obligately Anaerobic Bacteria"(PDF).Anti-Infective Agents in Medicinal Chemistry.6(1): 49–56.doi:10.2174/187152107779314179.Archived(PDF)from the original on 16 June 2010.Retrieved6 May2011.
- ^Zhanel GG, Fontaine S, Adam H, Schurek K, Mayer M, Noreddin AM, et al. (2006). "A Review of New Fluoroquinolones: Focus on their Use in Respiratory Tract Infections".Treatments in Respiratory Medicine.5(6): 437–465.doi:10.2165/00151829-200605060-00009.PMID17154673.S2CID26955572.
- ^Sanofi-Aventis U.S. LLC(September 2008)."NegGram Caplets (nalidixic acid, USP)"(PDF).Food and Drug Administration.
- ^Wentland MP (1993). "In memoriam: George Y. Lesher, PhD". In Hooper DC, Wolfson JS (eds.).Quinolone antimicrobial agents.Vol. XIII–XIV (2nd ed.). Washington DC: American Society for Microbiology.
- ^Norris S, Mandell GL (1988).The quinolones: history and overview.San Diego: Academic Press Inc. pp. 1–22.
- ^abcd"FDA updates warnings for fluoroquinolone antibiotics".FDA.26 July 2016.
- ^"Fluoroquinolone Safety Labeling Changes"(PDF).FDA. 4 April 2017. Archived fromthe original(PDF)on 9 May 2017.
- ^"Briefing Information for the November 5, 2015 Joint Meeting of the Antimicrobial Drugs Advisory Committee (AMDAC) and the Drug Safety and Risk Management Advisory Committee (DSaRM)".Food and Drug Administration. Archived fromthe originalon 20 October 2015.Retrieved19 October2015.
- ^"FDA Briefing Document Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee, November 5, 2015"(PDF).FDA. Archived fromthe original(PDF)on 17 November 2015.
- ^"Transcript: Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee, November 5, 2015"(PDF).FDA. Archived fromthe original(PDF)on 8 February 2016.
- ^Burton TM (6 November 2015)."FDA Panel Seeks Tougher Antibiotic Labels".The Wall Street Journal.Retrieved6 November2015.
- ^Sanofi-Aventis U.S. LLC(September 2008)."NegGram® Caplets (nalidixic acid, USP)"(PDF).USA: FDA.Retrieved6 May2011.
- ^Wentland MP: In memoriam: George Y. Lesher, PhD, in Hooper DC, Wolfson JS (eds): Quinolone antimicrobial agents, ed 2., Washington DC, American Society for Microbiology: XIII – XIV, 1993.
- ^Ball P (July 2000)."Quinolone generations: natural history or natural selection?".The Journal of Antimicrobial Chemotherapy.46(Supplement 3): 17–24.doi:10.1093/oxfordjournals.jac.a020889.PMID10997595.
- ^Lilley SH, Malone R, King DE (May 2000)."New Classification and Update on the Quinolone Antibiotics – May 1, 2000 – American Academy of Family Physicians".American Family Physician.61(9): 2741–2748. Archived fromthe originalon 6 June 2011.Retrieved18 March2008.
- ^abcdefghijklOliphant CM, Green GM (February 2002)."Quinolones: a comprehensive review".American Family Physician.65(3): 455–464.PMID11858629.Archivedfrom the original on 29 September 2007.Retrieved6 May2011.
- ^abcdAmbrose PG, Owens Jr RC (1 March 2000)."Clinical Usefulness of Quinolones".Seminars in Respiratory and Critical Care Medicine.Archivedfrom the original on 23 June 2011.Retrieved6 May2011.
- ^"Consolidated list of products – Pharmaceuticals 12th issue"(PDF).United Nations. 2005.Retrieved6 May2011.
- ^Gupta (2009).Clinical Ophthalmology: Contemporary Perspectives, 9/e.Elsevier India. pp. 112–.ISBN978-81-312-1680-4.Retrieved20 September2010.
- ^Schmid RE (1 May 2006)."Drug Company Taking Tequin Off Market".Associated Press. Archived fromthe originalon 25 November 2007.Retrieved1 May2006.
External links
[edit]- Quinolone antibioticatCurlie
- Healthcare-associated Infections (HAIs)- Quinolones and the Clinical LaboratoryCDC
- Information for Healthcare Professionals: Fluoroquinolone Antimicrobial Drugsfrom theU.S. Food and Drug Administration
- FluoroquinolonesArchived17 June 2006 at theWayback Machine"Family Practice Notebook" entry page for Fluoroquinolones
- Structure Activity Relationships"Antibacterial Agents; Structure Activity Relationships," André Bryskier MD
