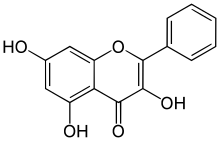
Galangin
Appearance
| |

| |
| Names | |
|---|---|
| IUPAC name
3,5,7-Trihydroxyflavone
| |
| Systematic IUPAC name
3,5,7-Trihydroxy-2-phenyl-4H-1-benzopyran-4-one | |
| Other names
Norizalpinin
3,5,7-triOH-Flavone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.147 |
| KEGG | |
PubChemCID
|
|
| UNII | |
CompTox Dashboard(EPA)
|
|
| |
| |
| Properties | |
| C15H10O5 | |
| Molar mass | 270.240g·mol−1 |
| Density | 1.579 g/mL |
| Melting point | 214 to 215 °C (417 to 419 °F; 487 to 488 K) |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
Galanginis aflavonol,a type offlavonoid.
Occurrence[edit]
Galangin is found in high concentrations in plants likeAlpinia officinarum(lesser galangal)[1]andHelichrysum aureonitens.[2]It is also found in therhizomeofAlpinia galanga[3]and inpropolis.[4]
Biological activities[edit]
Galangin has been shown to havein vitroantibacterial[5][6]andantiviralactivity.[7]It also inhibits the growth ofbreast tumorcellsin vitro.[8][9]
References[edit]
- ^Ciolino, H. P.; Yeh, G. C. (1999)."The flavonoid galangin is an inhibitor of CYP1A1 activity and an agonist/antagonist of the aryl hydrocarbon receptor".British Journal of Cancer.79(9/10): 1340–1346.doi:10.1038/sj.bjc.6690216.PMC2362711.PMID10188874.
- ^Afolayan AJ, Meyer JJ (1997). "The antimicrobial activity of 3,5,7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens".Journal of Ethnopharmacology.57(3): 177–181.doi:10.1016/s0378-8741(97)00065-2.PMID9292410.
- ^Kaur, A.; Singh, R.; Dey, C. S.; Sharma, S. S.; Bhutani, K. K.; Singh, I. P. (2010)."Antileishmanial phenylpropanoids fromAlpinia galanga(Linn.) Willd "(PDF).Indian Journal of Experimental Biology.48(3): 314–317.PMID21046987.
- ^Tosi, E; Re, E; Ortega, M; Cazzoli, A (2007). "Food preservative based on propolis: Bacteriostatic activity of propolis polyphenols and flavonoids upon Escherichia coli".Food Chemistry.104(3): 1025–1029.doi:10.1016/j.foodchem.2007.01.011.
- ^Cushnie TP, Lamb AJ (2006). "Assessment of the antibacterial activity of galangin against 4-quinolone resistant strains of Staphylococcus aureus".Phytomedicine.13(3): 187–191.doi:10.1016/j.phymed.2004.07.003.PMID16428027.
- ^Cushnie TP, Lamb AJ (2005). "Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss".Journal of Ethnopharmacology.101(1–3): 243–248.doi:10.1016/j.jep.2005.04.014.PMID15985350.
- ^Afolayan AJ, Meyer JJ, Taylor MB, Erasmus D (1997). "Antiviral activity of galangin isolated from the aerial parts of Helichrysum aureonitens".Journal of Ethnopharmacology.56(2): 165–169.doi:10.1016/s0378-8741(97)01514-6.PMID917497.
- ^So, F. V.; Guthrie, N.; Chambers, A. F.; Moussa, M.; Carroll, K. K. (1996). "Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices".Nutrition and Cancer.26(2): 167–181.doi:10.1080/01635589609514473.PMID8875554.
- ^So, F.; Guthrie, N.; Chambers, A. F.; Carroll, K. K. (1997). "Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen".Cancer Letters.112(2): 127–133.doi:10.1016/S0304-3835(96)04557-0.PMID9066718.
