Gastrulation
| Gastrulation | |
|---|---|
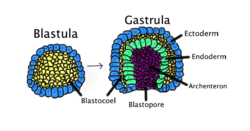 Gastrulation occurs when ablastula,made up of one layer, folds inward and enlarges to create a gastrula. This diagram is color-coded:ectoderm,blue;endoderm,green;blastocoel(the yolk sac), yellow; andarchenteron(the primary gut), purple. | |
| Identifiers | |
| MeSH | D054262 |
| Anatomical terminology | |
Gastrulationis the stage in the earlyembryonic developmentof mostanimals,during which theblastula(a single-layered hollow sphere ofcells), or in mammals theblastocyst,is reorganized into a two-layered or three-layered embryo known as thegastrula.[1]Before gastrulation, theembryois a continuousepithelialsheet of cells; by the end of gastrulation, the embryo has begundifferentiationto establish distinctcell lineages,set up the basic axes of the body (e.g.dorsal–ventral,anterior–posterior), and internalized one or more cell types including the prospectivegut.[2]
Gastrula layers[edit]
Intriploblasticorganisms, the gastrula is trilaminar (three-layered). These threegerm layersare theectoderm(outer layer),mesoderm(middle layer), andendoderm(inner layer).[3][4]Indiploblasticorganisms, such asCnidariaandCtenophora,the gastrula has only ectoderm and endoderm. The two layers are also sometimes referred to as thehypoblastandepiblast.[5]Spongesdo not go through the gastrula stage.
Gastrulation takes place aftercleavageand the formation of the blastula, or blastocyst. Gastrulation is followed byorganogenesis,when individualorgansdevelop within the newly formed germ layers.[6]Each layer gives rise to specifictissuesand organs in the developing embryo.
- The ectoderm gives rise toepidermis,thenervous system,and to theneural crestin vertebrates.[2]
- The endoderm gives rise to theepitheliumof thedigestive systemandrespiratory system,and organs associated with the digestive system, such as theliverandpancreas.[2]
- Themesodermgives rise to many cell types such asmuscle,bone,andconnective tissue.In vertebrates, mesoderm derivatives include thenotochord,theheart,bloodandblood vessels,thecartilageof theribsandvertebrae,and thedermis.[2][7]
Following gastrulation, cells in the body are either organized into sheets of connected cells (as inepithelia), or as a mesh of isolated cells, such asmesenchyme.[4][8]
Basic cell movements[edit]
Although gastrulation patterns exhibit enormous variation throughout the animal kingdom, they are unified by the five basic types of cell movements that occur during gastrulation:[2][9]
Etymology[edit]
The terms "gastrula" and "gastrulation" were coined byErnst Haeckel,in his 1872 work"Biology of Calcareous Sponges".[10] Gastrula (literally, "little belly" ) is a neo-Latin diminutive based on the Ancient Greekγαστήρgastḗr( "a belly" ).
Importance[edit]
Lewis Wolpert,pioneering developmental biologist in the field, has been credited for noting that "It is not birth, marriage, or death, but gastrulation which is truly the most important time in your life."[2][11]
Model systems[edit]
Gastrulation is highly variable across the animal kingdom but has underlying similarities. Gastrulation has been studied in many animals, but some models have been used for longer than others. Furthermore, it is easier to study development in animals that develop outside the mother.Model organismswhose gastrulation is understood in the greatest detail include themollusc,sea urchin,frog,andchicken.A human model system is thegastruloid.
Protostomes versus deuterostomes[edit]
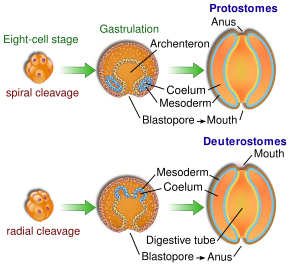
Thedistinction betweenprotostomesanddeuterostomesis based on the direction in which the mouth (stoma) develops in relation to theblastopore.Protostome derives from the Greek word protostoma meaning "first mouth" (πρῶτος + στόμα) whereas Deuterostome's etymology is "second mouth" from the words second and mouth (δεύτερος + στόμα).[citation needed]
The major distinctions between deuterostomes and protostomes are found inembryonic development:
- Mouth/anus
- Inprotostomedevelopment, the first opening in development, the blastopore, becomes the animal'smouth.
- Indeuterostomedevelopment, the blastopore becomes the animal'sanus.
- Cleavage
- Protostomeshave what is known asspiral cleavagewhich isdeterminate,meaning that the fate of the cells is determined as they are formed.
- Deuterostomeshave what is known asradial cleavagethat isindeterminate.
Sea urchins[edit]
Sea urchinshave been importantmodel organismsindevelopmental biologysince the 19th century.[12]Their gastrulation is often considered the archetype for invertebrate deuterostomes.[13]Experiments along with computer simulations have been used to gain knowledge about gastrulation in the sea urchin. Recent simulations found that planar cell polarity is sufficient to drive sea urchin gastrulation.[14]
Germ layer determination[edit]
Sea urchins exhibit highly stereotyped cleavage patterns and cell fates. Maternally depositedmRNAsestablish the organizing center of the sea urchin embryo. CanonicalWntandDelta-Notchsignaling progressively segregate progressive endoderm and mesoderm.[15]
Cell internalization[edit]
Insea urchinsthe first cells to internalize are the primarymesenchymecells (PMCs), which have askeletogenicfate, which ingress during the blastula stage. Gastrulation – internalization of the prospectiveendodermand non-skeletogenicmesoderm– begins shortly thereafter with invagination and other cell rearrangements thevegetal pole,which contribute approximately 30% to the finalarchenteronlength. Thegut's final lengthdepends on cell rearrangements within the archenteron.[16]
Amphibians[edit]
ThefroggenusXenopushas been used as amodel organismfor the study of gastrulation.[17]
Symmetry breaking[edit]
The sperm contributes one of the twomitotic astersneeded to complete first cleavage. The sperm can enter anywhere in theanimal halfof the egg but its exact point of entry will break the egg's radial symmetry by organizing thecytoskeleton.Prior to first cleavage, the egg's cortex rotates relative to the internalcytoplasmby the coordinated action ofmicrotubules,in a process known as cortical rotation. This displacement brings maternally loaded determinants of cell fate from the equatorial cytoplasm and vegetal cortex into contact, and together these determinants set up theorganizer.Thus, the area on the vegetal side opposite the sperm entry point will become the organizer.[18]Hilde Mangold,working in the lab ofHans Spemann,demonstrated that this special "organizer" of the embryo isnecessary and sufficientto induce gastrulation.[19][20][21]
Germ layer determination[edit]
Specification of endoderm depends on rearrangement of maternally deposited determinants, leading to nuclearization ofBeta-catenin.Mesoderm isinducedby signaling from the presumptive endoderm to cells that would otherwise become ectoderm.[18]
Cell internalization[edit]
Thedorsal lipof the blastopore is the mechanical driver of gastrulation. The first sign of invagination seen in the frog is the dorsal lip.[citation needed]
Cell signaling[edit]
In the frog,Xenopus,one of the signals isretinoic acid(RA).[22]RA signaling in this organism can affect the formation of the endoderm and depending on the timing of the signaling, it can determine the fate whether its pancreatic, intestinal, or respiratory. Other signals such as Wnt and BMP also play a role in respiratory fate of theXenopusby activating cell lineage tracers.[22]
Amniotes[edit]
Overview[edit]
Inamniotes(reptiles, birds and mammals), gastrulation involves the creation of the blastopore, an opening into thearchenteron.Note that the blastopore is not an opening into theblastocoel,the space within theblastula,but represents a new inpocketing that pushes the existing surfaces of the blastula together. Inamniotes,gastrulation occurs in the following sequence: (1) theembryobecomesasymmetric;(2) theprimitive streakforms; (3) cells from theepiblastat theprimitive streakundergo anepithelial to mesenchymal transitionandingressat theprimitive streakto form thegerm layers.[7]
Symmetry breaking[edit]
In preparation for gastrulation, the embryo must become asymmetric along both theproximal-distal axisand theanteroposterior axis.The proximal-distal axis is formed when the cells of the embryo form the "egg cylinder", which consists of the extraembryonic tissues, which give rise to structures like theplacenta,at the proximal end and theepiblastat the distal end. Many signaling pathways contribute to this reorganization, includingBMP,FGF,nodal,andWnt.Visceral endoderm surrounds theepiblast.Thedistalvisceral endoderm (DVE) migrates to theanteriorportion of the embryo, forming theanterior visceral endoderm(AVE). This breaks anterior-posterior symmetry and is regulated bynodalsignaling.[7]

Germ layer determination[edit]
Theprimitive streakis formed at the beginning of gastrulation and is found at the junction between the extraembryonic tissue and theepiblaston the posterior side of the embryo and the site ofingression.[23]Formation of theprimitive streakis reliant uponnodalsignaling[7]in theKoller's sicklewithin the cells contributing to the primitive streak andBMP4signaling from the extraembryonic tissue.[23][24]Furthermore,Cer1andLefty1restrict the primitive streak to the appropriate location by antagonizingnodalsignaling.[25]The region defined as theprimitive streakcontinues to grow towards the distal tip.[7]
During the early stages of development, the primitive streak is the structure that will establishbilateral symmetry,determine the site of gastrulation and initiate germ layer formation.[26]To form the streak, reptiles, birds and mammals arrange mesenchymal cells along the prospective midline, establishing the first embryonic axis, as well as the place where cells will ingress and migrate during the process of gastrulation and germ layer formation.[27]The primitive streak extends through this midline and creates the antero-posterior body axis,[28]becoming the first symmetry-breaking event in theembryo,and marks the beginning of gastrulation.[29]This process involves the ingression of mesoderm and endoderm progenitors and their migration to their ultimate position,[28][30]where they will differentiate into the three germ layers.[27]The localization of the cell adhesion and signaling moleculebeta-cateninis critical to the proper formation of the organizer region that is responsible for initiating gastrulation.
Cell internalization[edit]
In order for the cells to move from theepitheliumof theepiblastthrough theprimitive streakto form a new layer, the cells must undergo anepithelial to mesenchymal transition(EMT) to lose their epithelial characteristics, such ascell–cell adhesion.FGFsignaling is necessary for proper EMT.FGFR1is needed for the up regulation ofSNAI1,which down regulatesE-cadherin,causing a loss of cell adhesion. Following the EMT, the cellsingressthrough theprimitive streakand spread out to form a new layer of cells or join existing layers.FGF8is implicated in the process of this dispersal from theprimitive streak.[25]
Cell signaling[edit]
There are certain signals that play a role in determination and formation of the three germ layers, such as FGF, RA, and Wnt.[22]In mammals such as mice, RA signaling can play a role in lung formation. If there is not enough RA, there will be an error in the lung production. RA also regulates the respiratory competence in this mouse model.[citation needed]
Cell signaling driving gastrulation[edit]
During gastrulation, the cells are differentiated into the ectoderm ormesendoderm,which then separates into the mesoderm and endoderm.[22]The endoderm and mesoderm form due to thenodal signaling.Nodal signaling uses ligands that are part ofTGFβfamily. These ligands will signal transmembrane serine/threonine kinase receptors, and this will then phosphorylateSmad2andSmad3.This protein will then attach itself toSmad4and relocate to the nucleus where the mesendoderm genes will begin to be transcribed. TheWnt pathwayalong withβ-cateninplays a key role in nodal signaling and endoderm formation.[31]Fibroblast growth factors(FGF), canonical Wnt pathway,bone morphogenetic protein(BMP), andretinoic acid(RA) are all important in the formation and development of the endoderm.[22]FGF are important in producing thehomeoboxgene which regulates early anatomical development. BMP signaling plays a role in the liver and promotes hepatic fate. RA signaling also induce homeobox genes such as Hoxb1 and Hoxa5. In mice, if there is a lack in RA signaling the mouse will not develop lungs.[22]RA signaling also has multiple uses in organ formation of the pharyngeal arches, the foregut, and hindgut.[22]
Gastrulationin vitro[edit]
There have been a number of attempts to understand the processes of gastrulation usingin vitrotechniques in parallel and complementary to studies in embryos, usually though the use of2D[32][33][34]and 3D cell (Embryonic organoids) culture techniques[35][36][37][38]usingembryonic stem cells(ESCs) orinduced pluripotent stem cells(iPSCs). These are associated with number of clear advantages in using tissue-culture based protocols, some of which include reducing the cost of associatedin vivowork (thereby reducing, replacing and refining the use of animals in experiments;the 3Rs), being able to accurately apply agonists/antagonists in spatially and temporally specific manner[36][37]which may be technically difficult to perform during Gastrulation. However, it is important to relate the observations in culture to the processes occurring in the embryo for context.
To illustrate this, the guided differentiation of mouse ESCs has resulted in generatingprimitive streak–like cells that display many of the characteristics of epiblast cells that traverse through the primitive streak[32](e.g. transientbrachyuryup regulation and the cellular changes associated with anepithelial to mesenchymal transition[32]), and human ESCs cultured on micro patterns, treated withBMP4,can generate spatial differentiation pattern similar to the arrangement of thegerm layersin the human embryo.[33][34]Finally, using 3Dembryoid body- andorganoid-based techniques, small aggregates of mouse ESCs (Embryonic Organoids, or Gastruloids) are able to show a number of processes of early mammalian embryo development such as symmetry-breaking, polarisation of gene expression, gastrulation-like movements, axial elongation and the generation of all three embryonic axes (anteroposterior, dorsoventral and left-right axes).[35][36][37][39]
Invitrofertilization occurs in a laboratory. The process of invitrofertilization is when mature eggs are removed from the ovaries and are placed in a cultured medium where they are fertilized by sperm. In the culture the embryo will form.[40]14 days after fertilization the primitive streak forms. The formation of the primitive streak has been known to some countries as "human individuality".[41]This means that the embryo is now a being itself, it is its own entity. The countries that believe this have created a 14-day rule in which it is illegal to study or experiment on a human embryo after the 14-day period invitro.Research has been conducted on the first 14 days of an embryo, but no known studies have been done after the 14 days.[42]With the rule in place, mice embryos are used understand the development after 14 days; however, there are differences in the development between mice and humans.
See also[edit]
- Blastocyst
- Deuterostome
- Fate mapping
- Primitive node
- Invagination
- Neurulation
- Protostome
- Vegetal rotation
References[edit]
Notes[edit]
- ^Urry, Lisa (2016).Campbell Biology(11th ed.). Pearson. p. 1047.ISBN978-0134093413.
- ^abcdefGilbert, Scott F.; Michael J. F. Barresi (2016).Developmental biology(Eleventh ed.). Sunderland, Massachusetts: Sinauer.ISBN978-1-60535-470-5.OCLC945169933.
- ^Mundlos 2009:p. 422
- ^abMcGeady, 2004: p. 34
- ^Jonathon M.W., Slack (2013).Essential Developmental Biology.West Sussex, UK: Wiley-Blackwell. p. 122.ISBN978-0-470-92351-1.
- ^Hall, 1998:pp. 132-134
- ^abcdeArnold & Robinson, 2009
- ^Hall, 1998:p. 177
- ^Gilbert, Scott F. (2000)."Figure 8.6, [Types of cell movements during...]".www.ncbi.nlm.nih.gov.Retrieved11 May2022.
- ^Ereskovsky 2010:p. 236
- ^Wolpert L(2008)The triumph of the embryo.Courier Corporation, page 12.ISBN978-0-486-46929-4
- ^Laubichler, M.D. and Davidson, E. H. (2008). "Boveri's long experiment: sea urchin merogones and the establishment of the role of nuclear chromosomes in development".Developmental Biology.314(1):1–11.doi:10.1016/j.ydbio.2007.11.024.
- ^McClay, David R.; Gross, J.M.; Range, Ryan; Peterson, R.E.; Bradham, Cynthia (2004). "Chapter 9: Sea Urchin Gastrulation". In Stern, Claudio D. (ed.).Gastrulation: From Cells to Embryos.Cold Spring Harbor Laboratory Press. pp. 123–137.ISBN978-0-87969-707-5.
- ^Nielsen, Bjarke Frost; Nissen, Silas Boye; Sneppen, Kim; Mathiesen, Joachim; Trusina, Ala (February 21, 2020)."Model to Link Cell Shape and Polarity with Organogenesis".iScience.23(2): 100830.Bibcode:2020iSci...23j0830N.doi:10.1016/j.isci.2020.100830.PMC6994644.PMID31986479.S2CID210934521.
- ^McClay, D. R. 2009. Cleavage and Gastrulation in Sea Urchin. eLS.doi:10.1002/9780470015902.a0001073.pub2
- ^Hardin J D (1990)."Context-sensitive cell behaviors during gastrulation"(PDF).Semin. Dev. Biol.1:335–345.
- ^Blum, Martin; Beyer, Tina; Weber, Thomas; Vick, Philipp; Andre, Philipp; Bitzer, Eva; Schweickert, Axel (June 2009)."Xenopus, an ideal model system to study vertebrate left-right asymmetry".Developmental Dynamics.238(6): 1215–1225.doi:10.1002/dvdy.21855.PMID19208433.S2CID39348233.
- ^abGilbert, Scott F. (2000)."Axis Formation in Amphibians: The Phenomenon of the Organizer, The Progressive Determination of the Amphibian Axes".Developmental Biology.Sinauer Associates.
- ^Gilbert, Scott F. (2000)."Figure 10.20, [Organization of a secondary axis...]".www.ncbi.nlm.nih.gov.Retrieved1 June2020.
- ^Spemann H., Mangold H. (1924). "Über Induktion von Embryonanlagen durch Implantation artfremder Organisatoren".Roux' Arch. F. Entw. Mech.100(3–4): 599–638.doi:10.1007/bf02108133.S2CID12605303.
- ^De Robertis Edward (2006)."Spemann's organizer and self-regulation in amphibian embryos".Nature Reviews Molecular Cell Biology.7(4): 296–302.doi:10.1038/nrm1855.PMC2464568.PMID16482093.
- ^abcdefgZorn A, Wells J (2009)."Vertebrate Endoderm Development and Organ Formation".Annu Rev Cell Dev Biol.25:221–251.doi:10.1146/annurev.cellbio.042308.113344.PMC2861293.PMID19575677.
- ^abTam & Behringer, 1997
- ^Catala, 2005:p. 1535
- ^abTam, P.P.; Loebel, D.A (2007). "Gene function in mouse embryogenesis: get set for gastrulation".Nat Rev Genet.8(5): 368–81.doi:10.1038/nrg2084.PMID17387317.S2CID138874.
- ^Sheng, Guojun; Arias, Alfonso Martinez; Sutherland, Ann (2021-12-03)."The primitive streak and cellular principles of building an amniote body through gastrulation".Science.374(6572): abg1727.doi:10.1126/science.abg1727.PMID34855481.S2CID244841366.
- ^abMikawa T, Poh AM, Kelly KA, Ishii Y, Reese DE (2004)."Induction and patterning of the primitive streak, an organizing center of gastrulation in the amniote".Dev Dyn.229(3): 422–32.doi:10.1002/dvdy.10458.PMID14991697.S2CID758473.
- ^abDowns KM. (2009)."The enigmatic primitive streak: prevailing notions and challenges concerning the body axis of mammals".BioEssays.31(8): 892–902.doi:10.1002/bies.200900038.PMC2949267.PMID19609969.
- ^Chuai M, Zeng W, Yang X, Boychenko V, Glazier JA, Weijer CJ (2006)."Cell movement during chick primitive streak formation".Dev. Biol.296(1): 137–49.doi:10.1016/j.ydbio.2006.04.451.PMC2556955.PMID16725136.
- ^Chuai M, Weijer CJ (2008). "The mechanisms underlying primitive streak formation in the chick embryo.".Current Topics in Developmental Biology.Vol. 81. pp. 135–56.doi:10.1016/S0070-2153(07)81004-0.ISBN978-0-12-374253-7.PMID18023726.
- ^Grapin-Botton, A.; Constam, D. (2007). "Evolution of the mechanisms and molecular control of endoderm formation".Mechanisms of Development.124(4): 253–78.doi:10.1016/j.mod.2007.01.001.PMID17307341.S2CID16552755.
- ^abcTurner, David A.; Rué, Pau; Mackenzie, Jonathan P.; Davies, Eleanor; Martinez Arias, Alfonso (2014-01-01)."Brachyury cooperates with Wnt/β-catenin signalling to elicit primitive-streak-like behaviour in differentiating mouse embryonic stem cells".BMC Biology.12:63.doi:10.1186/s12915-014-0063-7.ISSN1741-7007.PMC4171571.PMID25115237.
- ^abWarmflash, Aryeh; Sorre, Benoit; Etoc, Fred; Siggia, Eric D; Brivanlou, Ali H (2014)."A method to recapitulate early embryonic spatial patterning in human embryonic stem cells".Nature Methods.11(8): 847–854.doi:10.1038/nmeth.3016.PMC4341966.PMID24973948.
- ^abEtoc, Fred; Metzger, Jakob; Ruzo, Albert; Kirst, Christoph; Yoney, Anna; Ozair, M. Zeeshan; Brivanlou, Ali H.; Siggia, Eric D. (2016)."A Balance between Secreted Inhibitors and Edge Sensing Controls Gastruloid Self-Organization".Developmental Cell.39(3): 302–315.doi:10.1016/j.devcel.2016.09.016.PMC5113147.PMID27746044.
- ^abBrink, Susanne C. van den; Baillie-Johnson, Peter; Balayo, Tina; Hadjantonakis, Anna-Katerina; Nowotschin, Sonja; Turner, David A.; Arias, Alfonso Martinez (2014-11-15)."Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells".Development.141(22): 4231–4242.doi:10.1242/dev.113001.ISSN0950-1991.PMC4302915.PMID25371360.
- ^abcTurner, David Andrew; Glodowski, Cherise R.; Luz, Alonso-Crisostomo; Baillie-Johnson, Peter; Hayward, Penny C.; Collignon, Jérôme; Gustavsen, Carsten; Serup, Palle; Schröter, Christian (2016-05-13). "Interactions between Nodal and Wnt signalling Drive Robust Symmetry Breaking and Axial Organisation in Gastruloids (Embryonic Organoids)".bioRxiv10.1101/051722.
- ^abcTurner, David; Alonso-Crisostomo, Luz; Girgin, Mehmet; Baillie-Johnson, Peter; Glodowski, Cherise R.; Hayward, Penelope C.; Collignon, Jérôme; Gustavsen, Carsten; Serup, Palle (2017-01-31). "Gastruloids develop the three body axes in the absence of extraembryonic tissues and spatially localised signalling".bioRxiv10.1101/104539.
- ^Beccari, Leonardo; Moris, Naomi; Girgin, Mehmet; Turner, David A.; Baillie-Johnson, Peter; Cossy, Anne-Catherine; Lutolf, Matthias P.; Duboule, Denis; Arias, Alfonso Martinez (October 2018)."Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids".Nature.562(7726): 272–276.Bibcode:2018Natur.562..272B.doi:10.1038/s41586-018-0578-0.ISSN0028-0836.PMID30283134.S2CID52915553.
- ^Turner, David A.; Girgin, Mehmet; Alonso-Crisostomo, Luz; Trivedi, Vikas; Baillie-Johnson, Peter; Glodowski, Cherise R.; Hayward, Penelope C.; Collignon, Jérôme; Gustavsen, Carsten (2017-11-01)."Anteroposterior polarity and elongation in the absence of extra-embryonic tissues and of spatially localised signalling in gastruloids: mammalian embryonic organoids".Development.144(21): 3894–3906.doi:10.1242/dev.150391.ISSN0950-1991.PMC5702072.PMID28951435.
- ^"In vitro fertilization (IVF) - Mayo Clinic".www.mayoclinic.org.Retrieved2022-04-11.
- ^Asplund, Kjell (2020)."Use of in vitro fertilization—ethical issues".Upsala Journal of Medical Sciences.125(2): 192–199.doi:10.1080/03009734.2019.1684405.ISSN2000-1967.PMC7721055.PMID31686575.S2CID207896932.
- ^Davis, Caitlin (2019-03-01). "The Boundaries of Embryo Research: Extending the Fourteen-Day Rule".Journal of Bioethical Inquiry.16(1): 133–140.doi:10.1007/s11673-018-09895-w.ISSN1872-4353.PMID30635823.S2CID58643344.
Bibliography[edit]
- Arnold, Sebastian J.;Robertson, Elizabeth J.(2009). "Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo".Nat. Rev. Mol. Cell Biol.10(2): 91–103.doi:10.1038/nrm2618.PMID19129791.S2CID94174.

- Catala, Martin (2005)."Embryology of the Spine and Spinal Cord".In Tortori-Donati, Paolo; et al. (eds.).Pediatric Neuroradiology: Brain.Springer.ISBN978-3-540-41077-5.
- Ereskovsky, Alexander V. (2010).The Comparative Embryology of Sponges.Springer.ISBN978-90-481-8574-0.
- Gilbert, Scott F. (2010).Developmental Biology(Ninth ed.). Sinauer Associates.ISBN978-0-87893-558-1.
- Hall, Brian Keith(1998)."8.3.3 The gastrula and gastrulation".Evolutionary developmental biology(2nd ed.). The Netherlands: Kluwer Academic Publishers.ISBN978-0-412-78580-1.
- Harrison, Lionel G.(2011).The Shaping of Life: The Generation of Biological Pattern.Cambridge University Press.ISBN978-0-521-55350-6.
- McGeady, Thomas A., ed. (2006). "Gastrulation".Veterinary embryology.Wiley-Blackwell.ISBN978-1-4051-1147-8.
- Mundlos, Stefan (2009). "Gene action: developmental genetics". In Speicher, Michael; et al. (eds.).Vogel and Motulsky's Human Genetics: Problems and Approaches(4th ed.). Springer.doi:10.1007/978-3-540-37654-5.ISBN978-3-540-37653-8.
- Tam, Patrick P.L.; Behringer, Richard R. (1997)."Mouse gastrulation: the formation of a mammalian body plan".Mech. Dev.68(1–2): 3–25.doi:10.1016/S0925-4773(97)00123-8.PMID9431800.S2CID14052942.

Further reading[edit]
- Baron, Margaret H. (2001)."Embryonic Induction of Mammalian Hematopoiesis and Vasculogenesis".In Zon, Leonard I. (ed.).Hematopoiesis: a developmental approach.Oxford University Press.ISBN978-0-19-512450-7.
- Cullen, K.E. (2009)."embryology and early animal development".Encyclopedia of life science, Volume 2.Infobase.ISBN978-0-8160-7008-4.
- Forgács, G.; Newman, Stuart A. (2005)."Cleavage and blastula formation".Biological physics of the developing embryo.Cambridge University Press.Bibcode:2005bpde.book.....F.ISBN978-0-521-78337-8.
- Forgács, G.; Newman, Stuart A. (2005)."Epithelial morphogenesis: gastrulation and neurulation".Biological physics of the developing embryo.Cambridge University Press.Bibcode:2005bpde.book.....F.ISBN978-0-521-78337-8.
- Hart, Nathan H.; Fluck, Richard A. (1995)."Epiboly and Gastrulation".In Capco, David (ed.).Cytoskeletal mechanisms during animal development.Academic Press.ISBN978-0-12-153131-7.
- Knust, Elizabeth (1999)."Gastrulation movements".In Birchmeier, Walter; Birchmeier, Carmen (eds.).Epithelial Morphogenesis in Development and Disease.CRC Press. pp. 152–153.ISBN978-90-5702-419-1.
- Kunz, Yvette W. (2004)."Gastrulation".Developmental biology of Teleost fishes.Springer.ISBN978-1-4020-2996-7.
- Nation, James L., ed. (2009)."Gastrulation".Insect physiology and biochemistry.CRC Press.ISBN978-0-8493-1181-9.
- Ross, Lawrence M.; Lamperti, Edward D., eds. (2006)."Human Ontogeny: Gastrulation, Neurulation, and Somite Formation".Atlas of anatomy: general anatomy and musculoskeletal system.Thieme.ISBN978-3-13-142081-7.
- Sanes, Dan H.; et al. (2006)."Early embryology of metazoans".Development of the nervous system(2nd ed.). Academic Press. pp. 1–2.ISBN978-0-12-618621-5.
- Stanger, Ben Z.; Melton, Douglas A. (2004)."Development of Endodermal Derivatives in the Lungs, Liver, Pancreas, and Gut".In Epstein, Charles J.; et al. (eds.).Inborn errors of development: the molecular basis of clinical disorders of morphogenesis.Oxford University Press.ISBN978-0-19-514502-1.
