Gene drive
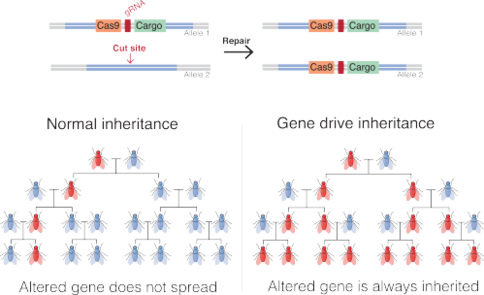
Agene driveis a natural process[1]and technology ofgenetic engineeringthat propagates a particular suite of genes throughout a population[2]by altering the probability that a specific allele will be transmitted to offspring (instead of theMendelian 50%probability). Gene drives can arise through a variety of mechanisms.[3][4]They have been proposed to provide an effective means of genetically modifying specific populations and entire species.
The technique can employ adding, deleting, disrupting, or modifying genes.[5][6]
Proposed applications include exterminating insects that carry pathogens (notably mosquitoes that transmitmalaria,dengue,andzikapathogens), controllinginvasive species,or eliminatingherbicideorpesticide resistance.[7][5][8][9]
As with any potentially powerful technique, gene drives can be misused in a variety of ways or induceunintended consequences.For example, a gene drive intended to affect only a local population might spread across an entire species. Gene drives that eradicate populations of invasive species in their non-native habitats may have consequences for the population of the species as a whole, even in its native habitat. Any accidental return of individuals of the species to its original habitats, through natural migration, environmental disruption (storms, floods, etc.), accidental human transportation, or purposeful relocation, could unintentionally drive the species to extinction if the relocated individuals carried harmful gene drives.[10]
Gene drives can be built from many naturally occurringselfish genetic elementsthat use a variety of molecular mechanisms.[3]These naturally occurring mechanisms induce similarsegregation distortionin the wild, arising whenallelesevolve molecular mechanisms that give them a transmission chance greater than the normal 50%.
Most gene drives have been developed in insects, notably mosquitoes, as a way to control insect-borne pathogens. Recent developments designed gene drives directly in viruses, notablyherpesviruses.These viral gene drives can propagate a modification into the population of viruses, and aim to reduce the infectivity of the virus.[11][12]
Mechanism[edit]
Insexually-reproducingspecies, most genes are present in two copies (which can be the same or differentalleles), either one of which has a 50% chance of passing to a descendant. By biasing the inheritance of particular altered genes, synthetic gene drives could more effectively spread alterations through a population.[5][6]
Typically, scientists insert the gene drive into an organism's DNA along with the CRISPR-Cas9 machinery. When the modified organism mates and its DNA mixes with that of its mate, the CRISPR-Cas9 tool cuts the partner's DNA at the same spot where the gene drive is located in the first organism. The cell repairs the cut DNA by copying the gene drive from the first organism into the corresponding spot in the DNA of the offspring. This means both copies of the gene (one from each parent) now contain the gene drive.
Molecular mechanisms[edit]
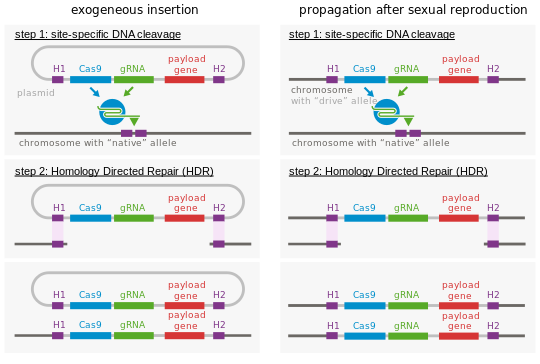
At the molecular level, an endonuclease gene drive works by cutting achromosomeat a specific site that does not encode the drive, inducing the cell torepair the damageby copying the drive sequence onto the damaged chromosome. The cell then has two copies of the drive sequence. The method derives fromgenome editingtechniques and relies onhomologous recombination.To achieve this behavior, endonuclease gene drives consist of two nested elements:
- Anendonucleasethat selectively cuts at the "target sequence", i.e. the rival allele. This can be one of:
- Ahoming endonuclease,which is what naturalinteinsuse to propagate. They are, however, very difficult, if not impossible, to retarget.[5]
- An RNA-guided endonuclease (e.g.,Cas9orCas12a[13]) and itsguide RNA,which can be easily altered to set the target.[5]Cas9 is the most promising technology identified in a 2014 review.[5]Cas9 gene drives have been successfully tested in 2015,[14]and Cas12a in 2023.[15]
- Any other programmable endonuclease system, such as modularzinc fingernucleases andTALEN.[5]One such drive has been successfully tested in fruit flies, but it turned out to be evolutionarily unstable due to the many-repeat nature of those endonucleases.[5]
- Atemplate sequenceused by the DNA repair machinery after the target sequence is cut. To achieve the self-propagating nature of gene drives, this repair template contains at least the endonuclease sequence. Because the template must be used to repair adouble-strand breakat the cutting site, its sides arehomologousto the sequences that are adjacent to the cutting site in the host genome. By targeting the gene drive to a gene coding sequence, this gene will be inactivated; additional sequences can be introduced in the gene drive to encode new functions.
As a result, the gene drive insertion in the genome will re-occur in each organism that inherits one copy of the modification and one copy of the wild-type gene. If the gene drive is already present in the egg cell (e.g. when received from one parent), all the gametes of the individual will carry the gene drive (instead of 50% in the case of a normal gene).[5]
Spreading in the population[edit]
This sectionneeds additional citations forverification.(November 2017) |
Since it can never more than double in frequency with each generation, a gene drive introduced in a single individual typically requires dozens of generations to affect a substantial fraction of a population. Alternatively, releasing drive-containing organisms in sufficient numbers can affect the rest within a few generations; for instance, by introducing it in every thousandth individual, it takes only 12–15 generations to be present in all individuals.[16]Whether a gene drive will ultimately become fixed in a population and at which speed depends on its effect on individual fitness, on the rate of allele conversion, and on the population structure. In a well mixed population and with realistic allele conversion frequencies (≈90%), population genetics predicts that gene drives get fixed for a selection coefficient smaller than 0.3;[16]in other words, gene drives can be used to spread modifications as long as reproductive success is not reduced by more than 30%. This is in contrast with normal genes, which can only spread across large populations if they increase fitness.
Gene drive in viruses[edit]
Because the strategy usually relies on the simultaneous presence of an unmodified and a gene drive allele in the samecell nucleus,it had generally been assumed that a gene drive could only be engineered in sexually reproducing organisms, excludingbacteriaandviruses.However, during aviral infection,viruses can accumulate hundreds or thousands of genome copies in infected cells. Cells are frequently co-infected by multiple virions andrecombinationbetween viral genomes is a well-known and widespread source of diversity for many viruses. In particular,herpesvirusesare nuclear-replicatingDNA viruseswith large double-stranded DNA genomes and frequently undergo homologous recombination during their replication cycle.
These properties have enabled the design of a gene drive strategy that doesn't involve sexual reproduction, instead relying onco-infectionof a given cell by a naturally occurring and an engineered virus. Upon co-infection, the unmodified genome is cut and repaired by homologous recombination, producing new gene drive viruses that can progressively replace the naturally occurring population. Incell cultureexperiments, it was shown that a viral gene drive can spread into the viral population and strongly reduce the infectivity of the virus, which opens novel therapeutic strategies against herpesviruses.[11]
Technical limitations[edit]
Because gene drives propagate by replacing other alleles that contain a cutting site and the corresponding homologies, their application has been mostly limited to sexually reproducing species (because they arediploidorpolyploidand alleles are mixed at each generation). As a side effect, inbreeding could in principle be an escape mechanism, but the extent to which this can happen in practice is difficult to evaluate.[17]
Due to the number of generations required for a gene drive to affect an entire population, the time to universality varies according to the reproductive cycle of each species: it may require under a year for some invertebrates, but centuries for organisms with years-long intervals between birth andsexual maturity,such as humans.[18]Hence this technology is of most use in fast-reproducing species.
Effectiveness in real practice varies between techniques, especially by choice ofgermlinepromoter. Lin and Potter 2016 (a) discloses the promoter technologyhomology assisted CRISPR knockin(HACK) and Lin and Potter 2016 (b) demonstrates actual use, achieving a high proportion of altered progeny from each alteredDrosophilamother.[19]
Issues[edit]
Issues highlighted by researchers include:[20]
- Mutations: A mutation could happen mid-drive, which has the potential to allow unwanted traits to "ride along".
- Escape: Cross-breeding orgene flowpotentially allow a drive to move beyond its target population.
- Ecological impacts: Even when new traits' direct impact on a target is understood, the drive may have side effects on the surroundings.
TheBroad Instituteof MIT and Harvard added gene drives to a list of uses of gene-editing technology it doesn't think companies should pursue.[21][better source needed]
Bioethics concerns[edit]
Gene drives affect all future generations and represent the possibility of a larger change in a living species than has been possible before.[22]
In December 2015, scientists of major world academies called for a moratorium on inheritablehuman genomeedits that would affect the germline, including those related toCRISPR-Cas9technologies,[23]but supported continued basic research and gene editing that would not affect future generations.[24]In February 2016, British scientists were given permission by regulators to genetically modifyhuman embryosby using CRISPR-Cas9 and related techniques on condition that the embryos were destroyed in seven days.[25][26]In June 2016, the USNational Academies of Sciences, Engineering, and Medicinereleased a report on their "Recommendations for Responsible Conduct" of gene drives.[27]
A 2018 mathematical modelling studies suggest that despite preexisting and evolving gene drive resistance (caused by mutations at the cutting site), even an inefficient CRISPR "alteration-type" gene drive can achievefixationin small populations. With a small but non-zero amount of gene flow among many local populations, the gene drive can escape and convert outside populations as well.[28]
Kevin M. Esveltstated that an open conversation was needed around the safety of gene drives: "In our view, it is wise to assume that invasive and self-propagating gene drive systems are likely to spread to every population of the target species throughout the world. Accordingly, they should only be built to combat true plagues such as malaria, for which we have few adequate countermeasures and that offer a realistic path towards an international agreement to deploy among all affected nations.".[29]He moved to an open model for his own research on using gene drives to eradicateLyme diseaseinNantucketandMartha's Vineyard.[30]Esvelt and colleagues suggested that CRISPR could be used to save endangered wildlife from extinction. Esvelt later retracted his support for the idea, except for extremely hazardous populations such as malaria-carrying mosquitoes, and isolated islands that would prevent the drive from spreading beyond the target area.[31]
History[edit]
Austin Burt, anevolutionary geneticistatImperial College London,introduced the possibility of conducting gene drives based on natural homing endonucleaseselfish genetic elementsin 2003.[6]
Researchers had already shown that such genes couldact selfishlyto spread rapidly over successive generations. Burt suggested that gene drives might be used to prevent a mosquito population from transmitting themalaria parasiteor to crash a mosquito population. Gene drives based on homing endonucleases have been demonstrated in the laboratory intransgenicpopulations of mosquitoes[32]and fruit flies.[33][34]However, homing endonucleases are sequence-specific. Altering their specificity to target other sequences of interest remains a major challenge.[3]The possible applications of gene drive remained limited until the discovery of CRISPR and associated RNA-guided endonucleases such asCas9andCas12a.
In June 2014, theWorld Health Organization(WHO) Special Programme for Research and Training in Tropical Diseases[35]issued guidelines[36]for evaluating genetically modified mosquitoes. In 2013 theEuropean Food Safety Authorityissued a protocol[37]forenvironmental assessmentsof allgenetically modified organisms.
Funding[edit]
Target Malaria,a project funded by theBill and Melinda Gates Foundation,invested $75 million in gene drive technology. The foundation originally estimated the technology to be ready for field use by 2029 somewhere in Africa. However, in 2016 Gates changed this estimate to some time within the following two years.[38]In December 2017, documents released under theFreedom of Information Actshowed thatDARPAhad invested $100 million in gene drive research.[39]
Control strategies[edit]
Scientists have designed multiple strategies to maintain control over gene drives.[citation needed]
In 2020, researchers reported the development of two activeguide RNA-only elements that, according to their study, may enable halting or deleting gene drives introduced into populations in the wild withCRISPR-Cas9 gene editing.The paper's senior author cautions that the two neutralizing systems they demonstrated in cage trials "should not be used with afalse sense of securityfor field-implemented gene drives ".[40][41]
If elimination is not necessary, it may be desirable to intentionally preserve the target population at a lower level by using a less severe gene drive technology. This works by maintaining the semi-defective population indefinitely in the target area, thereby crowding out potential nearby, wild populations that would otherwise move back in to fill a void.[42]
CRISPR[edit]
CRISPR[43]is the leadinggenetic engineeringmethod.[44]In 2014, Esvelt and coworkers first suggested that CRISPR/Cas9 might be used to build gene drives.[5]In 2015, researchers reported successful engineering of CRISPR-based gene drives inSaccharomyces[45],Drosophila,[46]andmosquitoes.[47][48]They reported efficient inheritance distortion over successive generations, with one study demonstrating the spread of a gene into laboratory populations.[48]Drive-resistant alleles were expected to arise for each of the described gene drives; however, this could be delayed or prevented by targeting highly conserved sites at which resistance was expected to have a severe fitness cost.
Because of CRISPR's targeting flexibility, gene drives could theoretically be used to engineer almost any trait. Unlike previous approaches, they could be tailored to block the evolution of drive resistance by targeting multiple sequences. CRISPR could also enable gene drive architectures that control rather than eliminate populations.[citation needed]
In 2022, t-CRISPR, was used to pass the “t haplotype” gene to about 95% of offspring. The approach spreads faulty copies of a female fertility gene to offspring, rendering them infertile. The researchers reported that their models suggested that adding 256 altered animals to an island with a population of 200,000 mice would eliminate the population in about 25 years. The traditional approaches of poison and traps were not needed.[49]
Applications[edit]
Gene drives have two main classes of application, which have implications of different significance:
- introduce a genetic modification in laboratory populations; once a strain or a line carrying the gene drive has been produced, the drive can be passed to any other line by mating. Here, the gene drive is used to much more easily achieve a task that could be accomplished with other techniques.
- introduce a genetic modification in wild populations. Gene drives constitute a major development that makes possible previously unattainable changes.
Because of their unprecedented potential risk, safeguard mechanisms have been proposed and tested.[45][50]
Disease vector species[edit]
One possible application is to genetically modifymosquitoes,mice,and other disease vectors so that they cannot transmit diseases, such asmalariaanddengue feverin the case of mosquitoes, andtick-borne diseasein the case of mice.[51]Researchers have claimed that by applying the technique to 1% of the wild population of mosquitoes, that they could eradicate malaria within a year.[52]
Invasive species control[edit]
A gene drive could be used to eliminateinvasive speciesand has, for example, been proposed as a way to eliminateinvasive species in New Zealand.[53]Gene drives for biodiversity conservation purposes are being explored as part of The Genetic Biocontrol of Invasive Rodents (GBIRd) program because they offer the potential for reduced risk to non-target species and reduced costs when compared to traditional invasive species removal techniques. Given the risks of such an approach described below, the GBIRd partnership is committed to a deliberate, step-wise process that will only proceed with public alignment, as recommended by the world's leading gene drive researchers from the Australian and US National Academy of Sciences and many others.[54]A wider outreach network for gene drive research exists to raise awareness of the value of gene drive research for the public good.[55]
Some scientists are concerned about the technique, fearing it could spread and wipe out species in native habitats.[56]The gene could mutate, potentially causing unforeseen problems (as could any gene).[57]Many non-native species can hybridize with native species, such that a gene drive afflicting a non-native plant or animal that hybridizes with a native species could doom the native species. Many non-native species have naturalized into their new environment so well that crops and/or native species have adapted to depend on them.[58]
Predator Free 2050[edit]
The Predator Free 2050 project is a New Zealand government program to eliminate eight invasive mammalian predator species (including rats, short-tailed weasels, and possums) from the country by 2050.[59][60]The project was first announced in 2016 by New Zealand's prime ministerJohn Keyand in January 2017 it was announced that gene drives would be considered in the effort, but this has not yet been actualised.[60]In 2017, one group in Australia and another in Texas released preliminary research into creating 'daughterless mice' using gene drives in mammals.[61]
California[edit]
In 2017, scientists at the University of California, Riverside developed a gene drive to attack theinvasivespotted-wing drosophila,a type of fruit fly native to Asia that is costing California's cherry farms $700 million per year because of its tail's razor-edgedovipositorthat destroys unblemished fruit. The primary alternative control strategy involves the use ofinsecticidescalledpyrethroidsthat kill almost all insects that it contacts.[21]
Wild animal welfare[edit]
The transhumanist philosopherDavid Pearcehas advocated for using CRISPR-based gene drives to reduce thesuffering of wild animals.[62]Kevin M. Esvelt,an American biologist who has helped develop gene drive technology, has argued that there is a moral case for the elimination of theNew World screwwormthrough such technologies because of the immense suffering that infested wild animals experience when they are eaten alive.[63]
See also[edit]
- Biological machines
- Cas9
- Cas12a
- Meiotic drive
- Genome editing
- Population control
- Sterile insect technique
- Synthetic biology
- Target Malaria
References[edit]
- ^Alphey, Luke S.; Crisanti, Andrea; Randazzo, Filippo (Fil); Akbari, Omar S. (2020-11-18)."Opinion: Standardizing the definition of gene drive".Proceedings of the National Academy of Sciences.117(49): 30864–30867.doi:10.1073/pnas.2020417117.ISSN0027-8424.PMC7733814.PMID33208534.
- ^Callaway E (21 July 2017)."US defence agencies grapple with gene drives".Nature.Retrieved2018-04-24.
- ^abcChamper J, Buchman A, Akbari OS (March 2016)."Cheating evolution: engineering gene drives to manipulate the fate of wild populations".Nature Reviews. Genetics.17(3): 146–59.doi:10.1038/nrg.2015.34.PMID26875679.
- ^Leftwich PT, Edgington MP, Harvey-Samuel T, Carabajal Paladino LZ, Norman VC, Alphey L (October 2018)."Recent advances in threshold-dependent gene drives for mosquitoes".Biochemical Society Transactions.46(5): 1203–1212.doi:10.1042/BST20180076.PMC6195636.PMID30190331.
- ^abcdefghijEsvelt KM,Smidler AL,Catteruccia F,Church GM (July 2014)."Concerning RNA-guided gene drives for the alteration of wild populations".eLife.3:e03401.doi:10.7554/eLife.03401.PMC4117217.PMID25035423.
- ^abcBurt A (May 2003)."Site-specific selfish genes as tools for the control and genetic engineering of natural populations".Proceedings. Biological Sciences.270(1518): 921–8.doi:10.1098/rspb.2002.2319.PMC1691325.PMID12803906.
- ^"U.S. researchers call for greater oversight of powerful genetic technology | Science/AAAS | News".News.sciencemag.org. 17 July 2014.Retrieved2014-07-18.
- ^Benedict M, D'Abbs P, Dobson S, Gottlieb M, Harrington L, Higgs S, et al. (April 2008). "Guidance for contained field trials of vector mosquitoes engineered to contain a gene drive system: recommendations of a scientific working group".Vector Borne and Zoonotic Diseases.8(2): 127–66.doi:10.1089/vbz.2007.0273.PMID18452399.
- ^Redford KH, Brooks TM, Macfarlane NB, Adams JS (2019).Genetic frontiers for conservation...technical assessment.doi:10.2305/iucn.ch.2019.05.en.ISBN978-2-8317-1974-0.S2CID212870281.
- ^"This Gene-Editing Tech Might Be Too Dangerous To Unleash".Wired.
- ^abWalter, Marius; Verdin, Eric (2020-09-28)."Viral gene drive in herpesviruses".Nature Communications.11(1): 4884.Bibcode:2020NatCo..11.4884W.doi:10.1038/s41467-020-18678-0.ISSN2041-1723.PMC7522973.PMID32985507.
- ^"Gene Drives Could Kill Mosquitoes and Suppress Herpesvirus Infections".American Council on Science and Health.2020-09-30.Retrieved2020-10-07.
- ^"Even CRISPR".The Economist. ISSN 0013-0613. Retrieved 2016-05-03. Note: Cas12a was previously known as Cpf1. The latter name is used in this 2015 article.
- ^Hammond, Andrew; Galizi, Roberto; Kyrou, Kyros; Simoni, Alekos; Siniscalchi, Carla; Katsanos, Dimitris; Gribble, Matthew; Baker, Dean; Marois, Eric; Russell, Steven; Burt, Austin; Windbichler, Nikolai; Crisanti, Andrea; Nolan, Tony (January 2016)."A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae".Nature Biotechnology.34(1): 78–83.doi:10.1038/nbt.3439.PMC4913862.
- ^Sanz Juste, Sara; Okamoto, Emily M.; Nguyen, Christina; Feng, Xuechun; López Del Amo, Víctor (12 October 2023)."Next-generation CRISPR gene-drive systems using Cas12a nuclease".Nature Communications.14(1).doi:10.1038/s41467-023-42183-9.PMC10567717.
- ^abUnckless RL, Messer PW, Connallon T, Clark AG (October 2015)."Modeling the Manipulation of Natural Populations by the Mutagenic Chain Reaction".Genetics.201(2): 425–31.doi:10.1534/genetics.115.177592.PMC4596658.PMID26232409.
- ^Bull JJ (2016-04-02). "Lethal Gene Drive Selects Escape through Inbreeding".bioRxiv10.1101/046847.
- ^Oye KA, Esvelt K, Appleton E, Catteruccia F, Church G, Kuiken T, et al. (August 2014)."Biotechnology. Regulating gene drives".Science.345(6197): 626–8.Bibcode:2014Sci...345..626O.doi:10.1126/science.1254287.PMID25035410.
- ^Hay, Bruce A.; Oberhofer, Georg; Guo, Ming (2021-01-07)."Engineering the Composition and Fate of Wild Populations with Gene Drive".Annual Review of Entomology.66(1).Annual Reviews:407–434.doi:10.1146/annurev-ento-020117-043154.ISSN0066-4170.PMID33035437.S2CID222257628.
- ^Drinkwater K, Kuiken T, Lightfoot S, McNamara J, Oye K (May 2014)."Creating a research agenda for the ecological implications of synthetic biology".Cambridge, MA and Washington, DC.: MIT Center for International Studies and Woodrow Wilson International Center for Scholars. Archived fromthe original(PDF)on 2014-07-30.Retrieved2014-07-20.
- ^abRegalado A (December 12, 2017)."California farmers are eyeing a controversial genetic tool to eliminate fruit flies".MIT Technology Review.Retrieved2018-04-28.
- ^"Genetically Engineering Almost Anything".PBS. 17 July 2014."I don't care if it's a weed or a blight, people still are going to say this is way too massive a genetic engineering project," [bioethicist] Caplan says. "Secondly, it's altering things that are inherited, and that's always been a bright line for genetic engineering."
- ^Wade N(3 December 2015)."Scientists Place Moratorium on Edits to Human Genome That Could Be Inherited".The New York Times.Retrieved3 December2015.
- ^Huffaker S (9 December 2015)."Geneticists vote to allow gene editing of human embryos".New Scientist.Retrieved18 March2016.
- ^Gallagher J (1 February 2016)."Scientists get 'gene editing' go-ahead".BBC News.Retrieved1 February2016.
- ^Cheng M (1 February 2016)."Britain approves controversial gene-editing technique".Associated Press.Archived fromthe originalon 1 February 2016.Retrieved1 February2016.
- ^"Gene Drive Research in Non-Human Organisms: Recommendations for Responsible Conduct".National Academies of Sciences, Engineering, and Medicine.June 8, 2016.RetrievedJune 9,2016.
- ^Noble C, Adlam B, Church GM, Esvelt KM, Nowak MA (June 2018)."Current CRISPR gene drive systems are likely to be highly invasive in wild populations".eLife.7.doi:10.7554/eLife.33423.PMC6014726.PMID29916367.
- ^Esvelt KM, Gemmell NJ (November 2017)."Conservation demands safe gene drive".PLOS Biology.15(11): e2003850.doi:10.1371/journal.pbio.2003850.PMC5689824.PMID29145398.
- ^Yong E (11 July 2017)."One Man's Plan to Make Sure Gene Editing Doesn't Go Haywire".theatlantic.com.Retrieved13 December2017.
- ^Zimmer C (2017-11-16)."'Gene Drives' Are Too Risky for Field Trials, Scientists Say ".The New York Times.ISSN0362-4331.Retrieved2018-04-22.
- ^Windbichler N, Menichelli M, Papathanos PA, Thyme SB, Li H, Ulge UY, et al. (May 2011)."A synthetic homing endonuclease-based gene drive system in the human malaria mosquito".Nature.473(7346): 212–5.Bibcode:2011Natur.473..212W.doi:10.1038/nature09937.PMC3093433.PMID21508956.
- ^Chan YS, Naujoks DA, Huen DS, Russell S (May 2011)."Insect population control by homing endonuclease-based gene drive: an evaluation in Drosophila melanogaster".Genetics.188(1): 33–44.doi:10.1534/genetics.111.127506.PMC3120159.PMID21368273.
- ^Chan YS, Huen DS, Glauert R, Whiteway E, Russell S (2013)."Optimising homing endonuclease gene drive performance in a semi-refractory species: the Drosophila melanogaster experience".PLOS ONE.8(1): e54130.Bibcode:2013PLoSO...854130C.doi:10.1371/journal.pone.0054130.PMC3548849.PMID23349805.
- ^"TDR | About us".Who.int.Retrieved2014-07-18.
- ^"TDR | A new framework for evaluating genetically modified mosquitoes".Who.int. 2014-06-26.Retrieved2014-07-18.
- ^"EFSA – Guidance of the GMO Panel: Guidance Document on the ERA of GM animals".EFSA Journal.11(5): 3200. 2013.doi:10.2903/j.efsa.2013.3200.hdl:10044/1/40807.Retrieved2014-07-18.
- ^Regalado A."Bill Gates doubles his bet on wiping out mosquitoes with gene editing".Retrieved2016-09-20.
- ^Neslen A (2017-12-04)."US military agency invests $100m in genetic extinction technologies".The Guardian.ISSN0261-3077.Retrieved2017-12-04.
- ^"Biologists create new genetic systems to neutralize gene drives".phys.org.Retrieved8 October2020.
- ^Xu, Xiang-Ru Shannon; Bulger, Emily A.; Gantz, Valentino M.; Klanseck, Carissa; Heimler, Stephanie R.; Auradkar, Ankush; Bennett, Jared B.; Miller, Lauren Ashley; Leahy, Sarah; Juste, Sara Sanz; Buchman, Anna; Akbari, Omar S.; Marshall, John M.; Bier, Ethan (18 September 2020)."Active Genetic Neutralizing Elements for Halting or Deleting Gene Drives".Molecular Cell.80(2): 246–262.e4.doi:10.1016/j.molcel.2020.09.003.ISSN1097-2765.PMC10962758.PMID32949493.S2CID221806864.
- ^Dhole, Sumit; Lloyd, Alun L.; Gould, Fred (2020-11-02)."Gene Drive Dynamics in Natural Populations: The Importance of Density Dependence, Space, and Sex".Annual Review of Ecology, Evolution, and Systematics.51(1).Annual Reviews:505–531.arXiv:2005.01838.doi:10.1146/annurev-ecolsys-031120-101013.ISSN1543-592X.PMC8340601.PMID34366722.
- ^Pennisi E(2013-08-23)."The CRISPR Craze".Science.341(6148). Sciencemag.org: 833–6.Bibcode:2013Sci...341..833P.doi:10.1126/science.341.6148.833.PMID23970676.Retrieved2014-07-18.
- ^Pollack A (May 11, 2015)."Jennifer Doudna, a Pioneer Who Helped Simplify Genome Editing".New York Times.RetrievedMay 12,2015.
- ^abDiCarlo JE, Chavez A, Dietz SL, Esvelt KM, Church GM (2015). "RNA-guided gene drives can efficiently and reversibly bias inheritance in wild yeast".bioRxiv10.1101/013896.
- ^Gantz VM, Bier E (April 2015)."Genome editing. The mutagenic chain reaction: a method for converting heterozygous to homozygous mutations".Science.348(6233): 442–4.doi:10.1126/science.aaa5945.PMC4687737.PMID25908821.
- ^Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E, James AA (December 2015)."Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi".Proceedings of the National Academy of Sciences of the United States of America.112(49): E6736-43.Bibcode:2015PNAS..112E6736G.doi:10.1073/pnas.1521077112.PMC4679060.PMID26598698.
- ^abHammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, et al. (January 2016)."A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae".Nature Biotechnology.34(1): 78–83.doi:10.1038/nbt.3439.PMC4913862.PMID26641531.
- ^Houser, Kristin (December 11, 2022)."New CRISPR tech makes it possible to wipe out invasive mice".Freethink.Retrieved2022-12-14.
- ^DiCarlo JE, Chavez A, Dietz SL, Esvelt KM, Church GM (December 2015)."Safeguarding CRISPR-Cas9 gene drives in yeast".Nature Biotechnology.33(12): 1250–1255.doi:10.1038/nbt.3412.PMC4675690.PMID26571100.
- ^Buchthal, Joanna; Evans, Sam Weiss; Lunshof, Jeantine; Telford, Sam R.; Esvelt, Kevin M. (2019-05-13)."Mice Against Ticks: an experimental community-guided effort to prevent tick-borne disease by altering the shared environment".Philosophical Transactions of the Royal Society B: Biological Sciences.374(1772): 20180105.doi:10.1098/rstb.2018.0105.PMC6452264.PMID30905296.
- ^Kahn J (2016-06-02).Gene editing can now change an entire species – forever.TED.
- ^Kalmakoff J (11 October 2016)."CRISPR for pest-free NZ".Retrieved19 October2016.
- ^"GBIRd Fact Sheet"(PDF).1 April 2018.Retrieved14 November2018.
- ^"Mission & Principles Statement".1 July 2018.Retrieved14 November2018.
- ^"'Gene drives' could wipe out whole populations of pests in one fell swoop ".THE CONVERSATION.
- ^"An Argument Against Gene Drives to Extinguish New Zealand Mammals: Life Finds a Way".Plos blogs.30 November 2017.
- ^Campbell C (17 October 2016)."Risks may accompany gene drive technology".Otago Daily Times.Retrieved19 October2016.
- ^Stockton N (July 27, 2016)."How New Zealand Plans to Kill Its (Non-Human) Invasive Mammals".WIRED.
- ^ab"Predator Free NZ – Expert Q&A".Scoop. 17 January 2017.Retrieved17 January2017.
- ^Regalado A (10 February 2017)."First Gene Drive in Mammals Could Aid Vast New Zealand Eradication Plan".MIT Tech Review.Retrieved14 February2017.
- ^Vinding M (2018-08-01)."Reducing Extreme Suffering for Non-Human Animals: Enhancement vs. Smaller Future Populations?".Between the Species.23(1).
- ^Esvelt K (2019-08-30)."When Are We Obligated To Edit Wild Creatures?".leapsmag.Retrieved2020-05-03.
Further reading[edit]
- Esvelt KM, Gemmell NJ (November 2017)."Conservation demands safe gene drive".PLOS Biology.15(11): e2003850.doi:10.1371/journal.pbio.2003850.PMC5689824.PMID29145398.
- Noble C, Adlam B, Church GM, Esvelt KM, Nowak MA (June 2018)."Current CRISPR gene drive systems are likely to be highly invasive in wild populations".eLife.7:219022.bioRxiv10.1101/219022.doi:10.7554/eLife.33423.002.PMC6014726.PMID29916367.S2CID196680955.
- De Chant T (July 17, 2014)."Genetically Engineering Almost Anything".NOVA.Retrieved11 August2014.
- Johnson C (July 17, 2014)."Harvard scientists want gene-manipulation debate".National Geographic.Retrieved11 August2014.
- Langin K (July 17, 2014)."Genetic Engineering to the Rescue Against Invasive Species?".National Geographic.Archived fromthe originalon July 27, 2014.Retrieved11 August2014.
- Zimmer C (July 17, 2014)."A Call to Fight Malaria One Mosquito at a Time by Altering DNA".The New York Times.Retrieved20 July2014.
- "The age of the red pen".The Economist.August 22, 2015.ISSN0013-0613.Retrieved2015-08-25.
- "The most selfish genes".The Economist.August 22, 2015.ISSN0013-0613.Retrieved2015-08-25.
- Esvelt K."Gene Drives for the Alteration of Wild Populations".Archived fromthe originalon 12 August 2014.Retrieved11 August2014.
