HIV
| Human immunodeficiency viruses | |
|---|---|

| |
| Scanning electron micrographof HIV-1 (in green) budding from culturedlymphocyte.Multiple round bumps on cell surface represent sites of assembly and budding of virions. | |
| Scientific classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Pararnavirae |
| Phylum: | Artverviricota |
| Class: | Revtraviricetes |
| Order: | Ortervirales |
| Family: | Retroviridae |
| Subfamily: | Orthoretrovirinae |
| Genus: | Lentivirus |
| Groups included | |
| Other lentiviruses | |
Thehuman immunodeficiency viruses(HIV) are two species ofLentivirus(a subgroup ofretrovirus) that infect humans. Over time, they causeacquired immunodeficiency syndrome(AIDS),[1][2]a condition in which progressive failure of theimmune systemallows life-threateningopportunistic infectionsandcancersto thrive.[3]Without treatment, the average survival time after infection with HIV is estimated to be 9 to 11 years, depending on theHIV subtype.[4]
In most cases, HIV is asexually transmitted infectionandoccursby contact with or transfer ofblood,pre-ejaculate,semen,andvaginal fluids.[5][6]Non-sexual transmission can occur from an infected mother to her infant duringpregnancy,duringchildbirthby exposure to her blood or vaginal fluid, and throughbreast milk.[7][8][9][10]Within these bodily fluids, HIV is present as both freevirusparticles and virus within infectedimmune cells. Research has shown (for both same-sex and opposite-sex couples) that HIV is untransmittable through condomless sexual intercourse if the HIV-positive partner has a consistently undetectableviral load.[5][6]
HIV infects vital cells in the human immune system, such ashelper T cells(specificallyCD4+T cells),macrophages,anddendritic cells.[11]HIV infection leads to low levels of CD4+T cells through a number of mechanisms, includingpyroptosisof abortively infected T cells,[12]apoptosisof uninfected bystander cells,[13]direct viral killing of infected cells, and killing of infected CD4+T cells byCD8+cytotoxic lymphocytesthat recognize infected cells.[14]When CD4+T cell numbers decline below a critical level,cell-mediated immunityis lost, and the body becomes progressively more susceptible to opportunistic infections, leading to the development of AIDS.
Virology
| Species | Virulence | Infectivity | Prevalence | Inferred origin |
|---|---|---|---|---|
| HIV-1 | High | High | Global | Common chimpanzee |
| HIV-2 | Lower | Low | West Africa | Sooty mangabey |
Classification
HIV is a member of thegenusLentivirus,[15]part of the familyRetroviridae.[16]Lentiviruses have manymorphologiesandbiologicalproperties in common. Many species are infected by lentiviruses, which are characteristically responsible for long-duration illnesses with a longincubation period.[17]Lentiviruses are transmitted assingle-stranded,positive-sense,envelopedRNA viruses.Upon entry into the target cell, the viralRNAgenomeis converted (reverse transcribed) into double-strandedDNAby a virally encoded enzyme,reverse transcriptase,that is transported along with the viral genome in the virus particle. The resulting viral DNA is then imported into thecell nucleusand integrated into the cellular DNA by a virally encoded enzyme,integrase,and hostco-factors.[18]Once integrated, the virus may becomelatent,allowing the virus and its host cell to avoid detection by the immune system, for an indeterminate amount of time.[19]The virus can remain dormant in the human body for up to ten years after primary infection; during this period the virus does not cause symptoms. Alternatively, the integrated viral DNA may betranscribed,producing new RNA genomes and viral proteins, using host cell resources, that are packaged and released from the cell as new virus particles that will begin the replication cycle anew.
Two types of HIV have been characterized: HIV-1 and HIV-2. HIV-1 is the virus that was initially discovered and termed both lymphadenopathy associated virus (LAV) and human T-lymphotropic virus 3 (HTLV-III). HIV-1 is morevirulentand moreinfectivethan HIV-2,[20]and is the cause of the majority of HIV infections globally. The lower infectivity of HIV-2, compared to HIV-1, implies that fewer of those exposed to HIV-2 will be infected per exposure. Due to its relatively poor capacity for transmission, HIV-2 is largely confined toWest Africa.[21]
Structure and genome

HIV is similar in structure to other retroviruses. It is roughly spherical[22]with a diameter of about 120nm,around 100,000 times smaller in volume than ared blood cell.[23]It is composed of two copies of positive-sensesingle-strandedRNAthat codes for the virus' ninegenesenclosed by a conicalcapsidcomposed of 2,000 copies of the viral proteinp24.[24]The single-stranded RNA is tightly bound to nucleocapsid proteins, p7, and enzymes needed for the development of the virion such asreverse transcriptase,proteases,ribonucleaseandintegrase.A matrix composed of the viral protein p17 surrounds the capsid ensuring the integrity of the virion particle.[24]
This is, in turn, surrounded by theviral envelope,that is composed of thelipid bilayertaken from the membrane of a human host cell when the newly formed virus particle buds from the cell. The viral envelope contains proteins from the host cell and relatively few copies of the HIV envelope protein,[24]which consists of a cap made of three molecules known asglycoprotein (gp) 120,and a stem consisting of threegp41molecules that anchor the structure into the viral envelope.[25][26]The envelope protein, encoded by the HIVenvgene, allows the virus to attach to target cells and fuse the viral envelope with the targetcell's membranereleasing the viral contents into the cell and initiating the infectious cycle.[25]

As the sole viral protein on the surface of the virus, the envelope protein is a major target forHIV vaccineefforts.[27]Over half of the mass of the trimeric envelope spike is N-linkedglycans.The density is high as the glycans shield the underlying viral protein from neutralisation by antibodies. This is one of the most densely glycosylated molecules known and the density is sufficiently high to prevent the normal maturation process of glycans during biogenesis in the endoplasmic and Golgi apparatus.[28][29]The majority of the glycans are therefore stalled as immature 'high-mannose' glycans not normally present on human glycoproteins that are secreted or present on a cell surface.[30]The unusual processing and high density means that almost all broadly neutralising antibodies that have so far been identified (from a subset of patients that have been infected for many months to years) bind to, or are adapted to cope with, these envelope glycans.[31]
The molecular structure of the viral spike has now been determined byX-ray crystallography[32]andcryogenic electron microscopy.[33]These advances in structural biology were made possible due to the development of stablerecombinantforms of the viral spike by the introduction of an intersubunitdisulphide bondand anisoleucinetoprolinemutation(radical replacementof an amino acid) in gp41.[34]The so-called SOSIPtrimersnot only reproduce the antigenic properties of the native viral spike, but also display the same degree of immature glycans as presented on the native virus.[35]Recombinant trimeric viral spikes are promising vaccine candidates as they display less non-neutralisingepitopesthan recombinant monomeric gp120, which act to suppress the immune response to target epitopes.[36]

The RNA genome consists of at least seven structural landmarks (LTR,TAR,RRE,PE, SLIP, CRS, and INS), and nine genes (gag,pol,andenv,tat,rev,nef,vif,vpr,vpu,and sometimes a tenthtev,which is a fusion oftat,envandrev), encoding 19 proteins. Three of these genes,gag,pol,andenv,contain information needed to make the structural proteins for new virus particles.[24]For example,envcodes for a protein called gp160 that is cut in two by a cellular protease to form gp120 and gp41. The six remaining genes,tat,rev,nef,vif,vpr,andvpu(orvpxin the case of HIV-2), are regulatory genes for proteins that control the ability of HIV to infect cells, produce new copies of virus (replicate), or cause disease.[24]
The twotatproteins (p16 and p14) aretranscriptional transactivatorsfor the LTRpromoteracting by binding the TAR RNA element. The TAR may also be processed intomicroRNAsthat regulate theapoptosisgenesERCC1andIER3.[37][38]Therevprotein (p19) is involved in shuttling RNAs from the nucleus and the cytoplasm by binding to theRRERNA element. Thevifprotein (p23) prevents the action ofAPOBEC3G(a cellular protein thatdeaminatescytidinetouridinein the single-stranded viral DNA and/or interferes with reverse transcription[39]). Thevprprotein (p14) arrestscell divisionatG2/M.Thenefprotein (p27) down-regulatesCD4(the major viral receptor), as well as theMHC class Iandclass IImolecules.[40][41][42]
Nefalso interacts withSH3 domains.Thevpuprotein (p16) influences the release of new virus particles from infected cells.[24]The ends of each strand of HIV RNA contain an RNA sequence called along terminal repeat(LTR). Regions in the LTR act as switches to control production of new viruses and can be triggered by proteins from either HIV or the host cell. ThePsi elementis involved in viral genome packaging and recognized bygagandrevproteins. The SLIP element (TTTTTT) is involved in theframeshiftin thegag-polreading framerequired to make functionalpol.[24]
Tropism

The termviral tropismrefers to the cell types a virus infects. HIV can infect a variety of immune cells such asCD4+T cells,macrophages,andmicroglial cells.HIV-1 entry to macrophages and CD4+T cells is mediated through interaction of the virion envelope glycoproteins (gp120) with the CD4 molecule on the target cells' membrane and also withchemokineco-receptors.[25][43]
Macrophage-tropic (M-tropic) strains of HIV-1, or non-syncytia-inducing strains (NSI; now called R5 viruses[44]) use theβ-chemokine receptor,CCR5,for entry and are thus able to replicate in both macrophages and CD4+T cells.[45]This CCR5 co-receptor is used by almost all primary HIV-1 isolates regardless of viral genetic subtype. Indeed, macrophages play a key role in several critical aspects of HIV infection. They appear to be the first cells infected by HIV and perhaps the source of HIV production when CD4+cells become depleted in the patient. Macrophages and microglial cells are the cells infected by HIV in thecentral nervous system.In thetonsilsandadenoidsof HIV-infected patients, macrophages fuse into multinucleatedgiant cellsthat produce huge amounts of virus.
T-tropic strains of HIV-1, orsyncytia-inducing strains (SI; now called X4 viruses[44]) replicate in primary CD4+T cells as well as in macrophages and use theα-chemokine receptor,CXCR4,for entry.[45][46][47]
Dual-tropic HIV-1 strains are thought to be transitional strains of HIV-1 and thus are able to use both CCR5 and CXCR4 as co-receptors for viral entry.
Theα-chemokineSDF-1,aligandfor CXCR4, suppresses replication of T-tropic HIV-1 isolates. It does this bydown-regulatingthe expression of CXCR4 on the surface of HIV target cells. M-tropic HIV-1 isolates that use only the CCR5 receptor are termed R5; those that use only CXCR4 are termed X4, and those that use both, X4R5. However, the use of co-receptors alone does not explain viral tropism, as not all R5 viruses are able to use CCR5 on macrophages for a productive infection[45]and HIV can also infect a subtype ofmyeloid dendritic cells,[48]which probably constitute areservoirthat maintains infection when CD4+T cell numbers have declined to extremely low levels.
Some people are resistant to certain strains of HIV.[49]For example, people with theCCR5-Δ32mutation are resistant to infection by the R5 virus, as the mutation leaves HIV unable to bind to this co-receptor, reducing its ability to infect target cells.
Sexual intercourseis the major mode of HIV transmission. Both X4 and R5 HIV are present in theseminal fluid,which enables the virus to be transmitted from a male to hissexual partner.The virions can then infect numerous cellular targets and disseminate into the whole organism. However, a selection process[further explanation needed]leads to a predominant transmission of the R5 virus through this pathway.[50][51][52]In patients infected with subtype B HIV-1, there is often a co-receptor switch in late-stage disease and T-tropic variants that can infect a variety of T cells through CXCR4.[53]These variants then replicate more aggressively with heightened virulence that causes rapid T cell depletion, immune system collapse, and opportunistic infections that mark the advent of AIDS.[54]HIV-positive patients acquire an enormously broad spectrum of opportunistic infections, which was particularly problematic prior to the onset ofHAARTtherapies; however, the same infections are reported among HIV-infected patients examined post-mortem following the onset of antiretroviral therapies.[3]Thus, during the course of infection, viral adaptation to the use of CXCR4 instead of CCR5 may be a key step in the progression to AIDS. A number of studies with subtype B-infected individuals have determined that between 40 and 50 percent of AIDS patients can harbour viruses of the SI and, it is presumed, the X4 phenotypes.[55][56]
HIV-2 is much less pathogenic than HIV-1 and is restricted in its worldwide distribution toWest Africa.The adoption of "accessory genes" by HIV-2 and its morepromiscuouspattern of co-receptor usage (including CD4-independence) may assist the virus in its adaptation to avoid innate restriction factors present in host cells. Adaptation to use normal cellular machinery to enable transmission and productive infection has also aided the establishment of HIV-2 replication in humans. A survival strategy for any infectious agent is not to kill its host, but ultimately become acommensalorganism. Having achieved a low pathogenicity, over time, variants that are more successful at transmission will be selected.[57]
Replication cycle
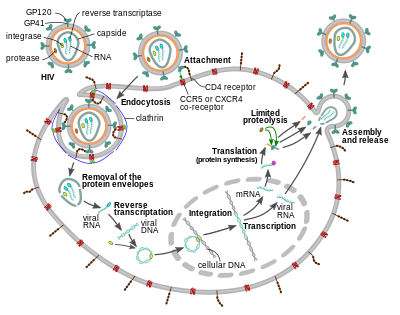
Entry to the cell

The HIV virion entersmacrophagesand CD4+T cellsby theadsorptionofglycoproteinson its surface to receptors on the target cell followed by fusion of theviral envelopewith the target cell membrane and the release of the HIV capsid into the cell.[58][59]
Entry to the cell begins through interaction of the trimeric envelope complex (gp160spike) on the HIV viral envelope and bothCD4and a chemokine co-receptor (generally eitherCCR5orCXCR4,but others are known to interact) on the target cell surface.[58][59]Gp120 binds tointegrinα4β7activatingLFA-1,the central integrin involved in the establishment ofvirological synapses,which facilitate efficient cell-to-cell spreading of HIV-1.[60]The gp160 spike contains binding domains for both CD4 and chemokine receptors.[58][59]
The first step in fusion involves the high-affinity attachment of the CD4 binding domains ofgp120to CD4. Once gp120 is bound with the CD4 protein, the envelope complex undergoes a structural change, exposing the chemokine receptor binding domains of gp120 and allowing them to interact with the target chemokine receptor.[58][59]This allows for a more stable two-pronged attachment, which allows theN-terminalfusion peptide gp41 to penetrate the cell membrane.[58][59]Repeat sequencesin gp41, HR1, and HR2 then interact, causing the collapse of the extracellular portion of gp41 into a hairpin shape. This loop structure brings the virus and cell membranes close together, allowing fusion of the membranes and subsequent entry of the viral capsid.[58][59]
After HIV has bound to the target cell, the HIV RNA and various enzymes, including reverse transcriptase, integrase, ribonuclease, and protease, are injected into the cell.[58][failed verification]During themicrotubule-based transport to the nucleus, the viral single-strand RNA genome is transcribed into double-strand DNA, which is then integrated into a host chromosome.
HIV can infectdendritic cells(DCs) by this CD4-CCR5 route, but another route usingmannose-specific C-type lectin receptorssuch asDC-SIGNcan also be used.[61]DCs are one of the first cells encountered by the virus during sexual transmission. They are currently thought to play an important role by transmitting HIV to T cells when the virus is captured in themucosaby DCs.[61]The presence ofFEZ-1,which occurs naturally inneurons,is believed to prevent the infection of cells by HIV.[62]

HIV-1 entry, as well as entry of many other retroviruses, has long been believed to occur exclusively at the plasma membrane. More recently, however, productive infection bypH-independent,clathrin-mediated endocytosisof HIV-1 has also been reported and was recently suggested to constitute the only route of productive entry.[63][64][65][66][67]
Replication and transcription

Shortly after the viral capsid enters the cell, anenzymecalledreverse transcriptaseliberates the positive-sense single-strandedRNAgenome from the attached viral proteins and copies it into acomplementary DNA(cDNA) molecule.[68]The process of reverse transcription is extremely error-prone, and the resulting mutations may causedrug resistanceor allow the virus to evade the body's immune system. The reverse transcriptase also has ribonuclease activity that degrades the viral RNA during the synthesis of cDNA, as well as DNA-dependent DNA polymerase activity that creates asenseDNA from theantisensecDNA.[69]Together, the cDNA and its complement form a double-stranded viral DNA that is then transported into thecell nucleus.The integration of the viral DNA into the host cell'sgenomeis carried out by another viral enzyme calledintegrase.[68]
The integrated viral DNA may then lie dormant, in the latent stage of HIV infection.[68]To actively produce the virus, certain cellulartranscription factorsneed to be present, the most important of which isNF-κB(nuclear factor kappa B), which is upregulated when T cells become activated.[70]This means that those cells most likely to be targeted, entered and subsequently killed by HIV are those actively fighting infection.
During viral replication, the integrated DNAprovirusistranscribedinto RNA. The full-length genomic RNAs (gRNA) can be packaged into new viral particles in apseudodiploidform. The selectivity in the packaging is explained by the structural properties of the dimeric conformer of the gRNA. The gRNA dimer is characterized by a tandem three-way junction within the gRNA monomer, in which the SD and AUGhairpins,responsible for splicing and translation respectively, are sequestered and the DIS (dimerization initiation signal) hairpin is exposed. The formation of the gRNA dimer is mediated by a 'kissing' interaction between the DIS hairpin loops of the gRNA monomers. At the same time, certain guanosine residues in the gRNA are made available for binding of the nucleocapsid (NC) protein leading to the subsequent virion assembly.[71]The labile gRNA dimer has been also reported to achieve a more stable conformation following the NC binding, in which both the DIS and the U5:AUG regions of the gRNA participate in extensive base pairing.[72]
RNA can also beprocessedto produce maturemessenger RNAs(mRNAs). In most cases, this processing involvesRNA splicingto produce mRNAs that are shorter than the full-length genome. Which part of the RNA is removed during RNA splicing determines which of the HIV protein-coding sequences is translated.[73]
Mature HIV mRNAs are exported from the nucleus into thecytoplasm,where they aretranslatedto produce HIV proteins, includingRev.As the newly produced Rev protein is produced it moves to the nucleus, where it binds to full-length, unspliced copies of virus RNAs and allows them to leave the nucleus.[74]Some of these full-length RNAs function as mRNAs that are translated to produce the structural proteins Gag and Env. Gag proteins bind to copies of the virus RNA genome to package them into new virus particles.[75] HIV-1 and HIV-2 appear to package their RNA differently.[76][77]HIV-1 will bind to any appropriate RNA.[78]HIV-2 will preferentially bind to the mRNA that was used to create the Gag protein itself.[79]
Recombination
Two RNA genomes are encapsidated in each HIV-1 particle (seeStructure and genome of HIV). Upon infection and replication catalyzed by reverse transcriptase, recombination between the two genomes can occur.[80][81]Recombination occurs as the single-strand, positive-sense RNA genomes are reverse transcribed to form DNA. During reverse transcription, the nascent DNA can switch multiple times between the two copies of the viral RNA. This form of recombination is known as copy-choice. Recombination events may occur throughout the genome. Anywhere from two to 20 recombination events per genome may occur at each replication cycle, and these events can rapidly shuffle the genetic information that is transmitted from parental to progeny genomes.[81]
Viral recombination produces genetic variation that likely contributes to theevolutionof resistance toanti-retroviral therapy.[82]Recombination may also contribute, in principle, to overcoming the immune defenses of the host. Yet, for the adaptive advantages of genetic variation to be realized, the two viral genomes packaged in individual infecting virus particles need to have arisen from separate progenitor parental viruses of differing genetic constitution. It is unknown how often such mixed packaging occurs under natural conditions.[83]
Bonhoefferet al.[84]suggested that template switching by reverse transcriptase acts as a repair process to deal with breaks in the single-stranded RNA genome. In addition, Hu and Temin[80]suggested that recombination is an adaptation for repair of damage in the RNA genomes. Strand switching (copy-choice recombination) by reverse transcriptase could generate an undamaged copy of genomic DNA from two damaged single-stranded RNA genome copies. This view of the adaptive benefit of recombination in HIV could explain why each HIV particle contains two complete genomes, rather than one. Furthermore, the view that recombination is a repair process implies that the benefit of repair can occur at each replication cycle, and that this benefit can be realized whether or not the two genomes differ genetically. On the view that recombination in HIV is a repair process, the generation of recombinational variation would be a consequence, but not the cause of, the evolution of template switching.[84]
HIV-1 infection causeschronic inflammationand production ofreactive oxygen species.[85]Thus, the HIV genome may be vulnerable tooxidative damage,including breaks in the single-stranded RNA. For HIV, as well as for viruses in general, successful infection depends on overcoming host defense strategies that often include production of genome-damaging reactive oxygen species. Thus, Michodet al.[86]suggested that recombination by viruses is an adaptation for repair of genome damage, and that recombinational variation is a byproduct that may provide a separate benefit.
Assembly and release
The final step of the viral cycle, assembly of new HIV-1 virions, begins at theplasma membraneof the host cell. The Env polyprotein (gp160) goes through theendoplasmic reticulumand is transported to theGolgi apparatuswhere it iscleavedbyfurinresulting in the two HIV envelope glycoproteins,gp41andgp120.[87]These are transported to the plasma membrane of the host cell where gp41 anchors gp120 to the membrane of the infected cell. The Gag (p55) and Gag-Pol (p160) polyproteins also associate with the inner surface of the plasma membrane along with the HIV genomic RNA as the forming virion begins to bud from the host cell. The budded virion is still immature as thegagpolyproteins still need to be cleaved into the actual matrix, capsid and nucleocapsid proteins. This cleavage is mediated by the packaged viral protease and can be inhibited by antiretroviral drugs of theprotease inhibitorclass. The various structural components then assemble to produce a mature HIV virion.[88]Only mature virions are then able to infect another cell.
Spread within the body

The classical process of infection of a cell by a virion can be called "cell-free spread" to distinguish it from a more recently recognized process called "cell-to-cell spread".[89]In cell-free spread (see figure), virus particles bud from an infected T cell, enter the blood orextracellular fluidand then infect another T cell following a chance encounter.[89]HIV can also disseminate by direct transmission from one cell to another by a process of cell-to-cell spread, for which two pathways have been described. Firstly, an infected T cell can transmit virus directly to a target T cell via avirological synapse.[60][90]Secondly, anantigen-presenting cell(APC), such as a macrophage or dendritic cell, can transmit HIV to T cells by a process that either involves productive infection (in the case of macrophages) or capture and transfer of virionsin trans(in the case of dendritic cells).[91]Whichever pathway is used, infection by cell-to-cell transfer is reported to be much more efficient than cell-free virus spread.[92]A number of factors contribute to this increased efficiency, including polarised virus budding towards the site of cell-to-cell contact, close apposition of cells, which minimizes fluid-phasediffusionof virions, and clustering of HIV entry receptors on the target cell towards the contact zone.[90]Cell-to-cell spread is thought to be particularly important inlymphoid tissues,where CD4+T cells are densely packed and likely to interact frequently.[89]Intravital imagingstudies have supported the concept of the HIV virological synapsein vivo.[93]The many dissemination mechanisms available to HIV contribute to the virus' ongoing replication in spite of anti-retroviral therapies.[89][94]
Genetic variability

HIV differs from many viruses in that it has very highgenetic variability.This diversity is a result of its fastreplication cycle,with the generation of about 1010virions every day, coupled with a highmutation rateof approximately 3 x 10−5pernucleotide baseper cycle of replication andrecombinogenicproperties of reverse transcriptase.[95][96][97]
This complex scenario leads to the generation of many variants of HIV in a single infected patient in the course of one day.[95]This variability is compounded when a single cell is simultaneously infected by two or more different strains of HIV. Whensimultaneous infectionoccurs, the genome of progeny virions may be composed of RNA strands from two different strains. This hybrid virion then infects a new cell where it undergoes replication. As this happens, the reverse transcriptase, by jumping back and forth between the two different RNA templates, will generate a newly synthesized retroviralDNA sequencethat is a recombinant between the two parental genomes.[95]This recombination is most obvious when it occurs between subtypes.[95]
The closely relatedsimian immunodeficiency virus(SIV) has evolved into many strains, classified by the natural host species. SIV strains of theAfrican green monkey(SIVagm) andsooty mangabey(SIVsmm) are thought to have a long evolutionary history with their hosts. These hosts have adapted to the presence of the virus,[98]which is present at high levels in the host's blood, but evokes only a mild immune response,[99]does not cause the development of simian AIDS,[100]and does not undergo the extensive mutation and recombination typical of HIV infection in humans.[101]
In contrast, when these strains infect species that have not adapted to SIV ( "heterologous" or similar hosts such asrhesusorcynomologus macaques), the animals develop AIDS and the virus generatesgenetic diversitysimilar to what is seen in human HIV infection.[102]ChimpanzeeSIV (SIVcpz), the closest genetic relative of HIV-1, is associated with increased mortality and AIDS-like symptoms in its natural host.[103]SIVcpz appears to have been transmitted relatively recently to chimpanzee and human populations, so their hosts have not yet adapted to the virus.[98]This virus has also lost a function of thenefgene that is present in most SIVs. For non-pathogenic SIV variants,nefsuppresses T cell activation through theCD3marker.Nef'sfunction in non-pathogenic forms of SIV is todownregulateexpression ofinflammatory cytokines,MHC-1,and signals that affect T cell trafficking. In HIV-1 and SIVcpz,nefdoes not inhibit T-cell activation and it has lost this function. Without this function, T cell depletion is more likely, leading to immunodeficiency.[103][104]
Three groups of HIV-1 have been identified on the basis of differences in the envelope (env) region: M, N, and O.[105]Group M is the most prevalent and is subdivided into eight subtypes (orclades), based on the whole genome, which are geographically distinct.[106]The most prevalent are subtypes B (found mainly in North America and Europe), A and D (found mainly in Africa), and C (found mainly in Africa and Asia); these subtypes form branches in thephylogenetic treerepresenting the lineage of the M group of HIV-1.Co-infectionwith distinct subtypes gives rise to circulating recombinant forms (CRFs). In 2000, the last year in which an analysis of global subtype prevalence was made, 47.2% of infections worldwide were of subtype C, 26.7% were of subtype A/CRF02_AG, 12.3% were of subtype B, 5.3% were of subtype D, 3.2% were of CRF_AE, and the remaining 5.3% were composed of other subtypes and CRFs.[107]Most HIV-1 research is focused on subtype B; few laboratories focus on the other subtypes.[108]The existence of a fourth group, "P", has been hypothesised based on a virus isolated in 2009.[109][110]The strain is apparently derived fromgorillaSIV (SIVgor), first isolated fromwestern lowland gorillasin 2006.[109]
HIV-2's closest relative is SIVsm, a strain of SIV found in sooty mangabees. Since HIV-1 is derived from SIVcpz, and HIV-2 from SIVsm, the genetic sequence of HIV-2 is only partially homologous to HIV-1 and more closely resembles that of SIVsm.[111][112]
Diagnosis
Many HIV-positive people are unaware that they are infected with the virus.[113]For example, in 2001 less than 1% of the sexually active urban population in Africa had been tested, and this proportion is even lower in rural populations.[113]Furthermore, in 2001 only 0.5% ofpregnant womenattending urban health facilities were counselled, tested or received their test results.[113]Again, this proportion is even lower in rural health facilities.[113]Since donors may therefore be unaware of their infection,donor bloodand blood products used in medicine andmedical researchare routinely screened for HIV.[114]
HIV-1 testing is initially done using anenzyme-linked immunosorbent assay(ELISA) to detect antibodies to HIV-1. Specimens with a non-reactive result from the initial ELISA are considered HIV-negative, unless new exposure to an infected partner or partner of unknown HIV status has occurred. Specimens with a reactive ELISA result are retested in duplicate.[115]If the result of either duplicate test is reactive, the specimen is reported as repeatedly reactive and undergoes confirmatory testing with a more specific supplemental test (e.g., apolymerase chain reaction(PCR),western blotor, less commonly, animmunofluorescence assay(IFA)). Only specimens that are repeatedly reactive by ELISA and positive by IFA or PCR or reactive by western blot are considered HIV-positive and indicative of HIV infection. Specimens that are repeatedly ELISA-reactive occasionally provide an indeterminate western blot result, which may be either an incomplete antibody response to HIV in an infected person or nonspecific reactions in an uninfected person.[116]
Although IFA can be used to confirm infection in these ambiguous cases, this assay is not widely used. In general, a second specimen should be collected more than a month later and retested for persons with indeterminate western blot results. Although much less commonly available,nucleic acid testing(e.g., viral RNA or proviral DNA amplification method) can also help diagnosis in certain situations.[115]In addition, a few tested specimens might provide inconclusive results because of a low quantity specimen. In these situations, a second specimen is collected and tested for HIV infection.
Modern HIV testing is extremely accurate, when thewindow periodis taken into consideration. A single screening test is correct more than 99% of the time.[118]The chance of a false-positive result in a standard two-step testing protocol is estimated to be about 1 in 250,000 in a low risk population.[119]Testing post-exposure is recommended immediately and then at six weeks, three months, and six months.[120]
The latest recommendations of the USCenters for Disease Control and Prevention(CDC) show that HIV testing must start with animmunoassaycombination test for HIV-1 and HIV-2antibodiesand p24antigen.A negative result rules out HIV exposure, while a positive one must be followed by an HIV-1/2 antibody differentiation immunoassay to detect which antibodies are present. This gives rise to four possible scenarios:
- 1. HIV-1 (+) & HIV-2 (−): HIV-1 antibodies detected
- 2. HIV-1 (−) & HIV-2 (+): HIV-2 antibodies detected
- 3. HIV-1 (+) & HIV-2 (+): both HIV-1 and HIV-2 antibodies detected
- 4. HIV-1 (−) or indeterminate & HIV-2 (−):Nucleic acid testmust be carried out to detect the acute infection of HIV-1 or its absence.[121]
Research
HIV/AIDS research includes allmedical researchthat attempts to prevent, treat, or cureHIV/AIDS,as well as fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
Many governments and research institutions participate in HIV/AIDS research. This research includes behavioralhealth interventions,such as research intosex education,anddrug development,such as research intomicrobicides for sexually transmitted diseases,HIV vaccines,andanti-retroviral drugs.[122]Other medical research areas include the topics ofpre-exposure prophylaxis,post-exposure prophylaxis,circumcision,andaccelerated aging effects.
Treatment and transmission
The management of HIV/AIDS normally includes the use of multipleantiretroviral drugs.In many parts of the world, HIV has become a chronic condition in which progression toAIDSis increasingly rare.
HIV latency, and the consequent viral reservoir in CD4+T cells, dendritic cells, as well as macrophages, is the main barrier to eradication of the virus.[19][123]
Although HIV is highly virulent, transmission does not occur through sex when an HIV-positive person has a consistently undetectableviral load(<50 copies/ml) due to anti-retroviral treatment. This was first argued by the Swiss Federal Commission for AIDS/HIV in 2008 in theSwiss Statement,though the statement was controversial at the time.[124][125]However, following multiple studies, it became clear that the chance of passing on HIV through sex is effectively zero where the HIV-positive person has a consistently undetectable viral load; this is known as U=U, "Undetectable=Untransmittable", also phrased as "can't pass it on".[126][127]The studies demonstrating U=U are: Opposites Attract,[128]PARTNER 1,[129]PARTNER 2,[5][130](for male-male couples)[131]and HPTN052[132](for heterosexual couples) when "the partner living with HIV had a durably suppressed viral load."[131]In these studies, couples where one partner was HIV positive and one partner was HIV negative were enrolled and regular HIV testing completed. In total from the four studies, 4097 couples were enrolled over four continents and 151,880 acts of condomless sex were reported; there were zero phylogenetically linked transmissions of HIV where the positive partner had an undetectable viral load.[133]Following this, the U=U consensus statement advocating the use of "zero risk" was signed by hundreds of individuals and organisations, including the USCDC,British HIV AssociationandThe Lancetmedical journal.[134]The importance of the final results of the PARTNER 2 study were described by the medical director of theTerrence Higgins Trustas "impossible to overstate", while lead author Alison Rodger declared that the message that "undetectable viral load makes HIV untransmittable... can help end the HIV pandemic by preventing HIV transmission.[135]The authors summarised their findings inThe Lancetas follows:[5]
Our results provide a similar level of evidence on viral suppression and HIV transmission risk for gay men to that previously generated for heterosexual couples and suggest that the risk of HIV transmission in gay couples through condomless sex when HIV viral load is suppressed is effectively zero. Our findings support the message of the U=U (undetectable equals untransmittable) campaign, and the benefits of early testing and treatment for HIV.[5]
This result is consistent with the conclusion presented byAnthony S. Fauci,the Director of theNational Institute of Allergy and Infectious Diseasesfor the U.S.National Institutes of Health,and his team in a viewpoint published in theJournal of the American Medical Association,that U=U is an effective HIV prevention method when an undetectable viral load is maintained.[6][131]
Herpes simplex virus-2(HSV-2) reactivation in those affected bygenital herpesis associated with an increase in CCR-5 enriched CD4+ T cells as well as inflammatory dendritic cells in the dermis of the ulcerated genital skin that persists after healing of the ulcer. Tropism of HIV for CCR-5 positive cells explains the two to threefold increase in HIV acquisition among persons with genital herpes. Daily antiviral (e.g. acyclovir) medication does not reduce the subclinical post-reactivation inflammation and therefore does not confer reduced risk of HIV acquisition.[136][137]
History
Discovery
The first news story on "an exotic new disease" appeared May 18, 1981, in the gay newspaperNew York Native.[138]
AIDS was first clinically observed in 1981 in the United States.[139]The initial cases were a cluster of injection drug users and gay men with no known cause of impaired immunity who showed symptoms ofPneumocystispneumonia (PCP or PJP, the latter term recognizing that the causative agent is now calledPneumocystis jirovecii), a rare opportunistic infection that was known to occur in people with very compromised immune systems.[140]Soon thereafter, researchers at theNYU School of Medicinestudied gay men developing a previously rare skin cancer calledKaposi's sarcoma(KS).[141][142]Many more cases of PJP and KS emerged, alerting U.S.Centers for Disease Control and Prevention(CDC) and a CDC task force was formed to monitor the outbreak.[143]The earliest retrospectively described case of AIDS is believed to have been in Norway beginning in 1966.[144]
In the beginning, the CDC did not have an official name for the disease, often referring to it by way of the diseases that were associated with it, for example,lymphadenopathy,the disease after which the discoverers of HIV originally named the virus.[145][146]They also usedKaposi's Sarcoma and Opportunistic Infections,the name by which a task force had been set up in 1981.[147]In the general press, the termGRID,which stood forgay-related immune deficiency,had been coined.[148]The CDC, in search of a name and looking at the infected communities, coined "the 4H disease", as it seemed to single out homosexuals, heroin users,hemophiliacs,andHaitians.[149][150]However, after determining that AIDS was not isolated to thegay community,[147]it was realized that the term GRID was misleading andAIDSwas introduced at a meeting in July 1982.[151]By September 1982 the CDC started using the name AIDS.[152]
In 1983, two separate research groups led by AmericanRobert Galloand French investigatorsFrançoise Barré-SinoussiandLuc Montagnierindependently declared that a novel retrovirus may have been infecting AIDS patients, and published their findings in the same issue of the journalScience.[153][146][154]Gallo claimed that a virus his group had isolated from a person with AIDS was strikingly similar inshapeto otherhuman T-lymphotropic viruses(HTLVs) his group had been the first to isolate. Gallo admitted in 1987 that the virus he claimed to have discovered in 1984 was in reality a virus sent to him from France the year before.[155]Gallo's group called their newly isolated virus HTLV-III. Montagnier's group isolated a virus from a patient presenting with swelling of thelymph nodesof the neck andphysical weakness,two classic symptoms of primary HIV infection. Contradicting the report from Gallo's group, Montagnier and his colleagues showed that core proteins of this virus were immunologically different from those of HTLV-I. Montagnier's group named their isolated virus lymphadenopathy-associated virus (LAV).[143]As these two viruses turned out to be the same, in 1986 LAV and HTLV-III were renamed HIV.[156]
Another group working contemporaneously with the Montagnier and Gallo groups was that ofJay A. Levyat theUniversity of California, San Francisco.He independently discovered the AIDS virus in 1983 and named it the AIDS associated retrovirus (ARV).[157]This virus was very different from the virus reported by the Montagnier and Gallo groups. The ARV strains indicated, for the first time, the heterogeneity of HIV isolates and several of these remain classic examples of the AIDS virus found in the United States.[158]
Origins
Both HIV-1 and HIV-2 are believed to have originated in non-humanprimatesin West-central Africa, and are believed to have transferred to humans (a process known aszoonosis) in the early 20th century.[159][160]
HIV-1 appears to have originated in southernCameroonthrough the evolution of SIVcpz, asimian immunodeficiency virus(SIV) that infects wildchimpanzees(HIV-1 descends from the SIVcpz endemic in the chimpanzee subspeciesPan troglodytes troglodytes).[161][162]The closest relative of HIV-2 is SIVsmm, a virus of thesooty mangabey(Cercocebus atys atys), anOld World monkeyliving in littoral West Africa (from southernSenegalto westernCôte d'Ivoire).[21]New World monkeyssuch as theowl monkeyare resistant to HIV-1 infection, possibly because of agenomic fusionof two viral resistance genes.[163]
HIV-1 is thought to have jumped the species barrier on at least three separate occasions, giving rise to the three groups of the virus, M, N, and O.[164]

There is evidence that humans who participate inbushmeatactivities, either as hunters or as bushmeat vendors, commonly acquire SIV.[165]However, SIV is a weak virus, and it is typically suppressed by the human immune system within weeks of infection. It is thought that several transmissions of the virus from individual to individual in quick succession are necessary to allow it enough time to mutate into HIV.[166]Furthermore, due to its relatively low person-to-person transmission rate, it can only spread throughout the population in the presence of one or more high-risk transmission channels, which are thought to have been absent in Africa prior to the 20th century.
Specific proposed high-risk transmission channels, allowing the virus to adapt to humans and spread throughout the society, depend on the proposed timing of the animal-to-human crossing. Genetic studies of the virus suggest that the most recent common ancestor of the HIV-1 M group dates back toc. 1910.[167]Proponents of this dating link the HIV epidemic with the emergence ofcolonialismand growth of large colonial African cities, leading to social changes, including different patterns of sexual contact (especially multiple, concurrent partnerships), the spread ofprostitution,and the concomitant high frequency ofgenital ulcerdiseases (such assyphilis) in nascent colonial cities.[168]While transmission rates of HIV during vaginal intercourse are typically low, they are increased manyfold if one of the partners has asexually transmitted infectionresulting in genital ulcers. Early 1900s colonial cities were notable for their high prevalence of prostitution and genital ulcers to the degree that as of 1928 as many as 45% of female residents of easternLeopoldville (currently Kinshasa)were thought to have been prostitutes and as of 1933 around 15% of all residents of the same city were infected by one of the forms ofsyphilis.[168]
The earliest, well-documented case of HIV in a human dates back to 1959 in theBelgian Congo.[169]The virus may have been present in the United States as early as the mid- to late 1960s, as a sixteen-year-old male namedRobert Rayfordpresented with symptoms in 1966 and died in 1969.[170]
An alternative and likely complementary hypothesis points to the widespread use of unsafe medical practices in Africa during years following World War II, such as unsterile reuse of single-use syringes during mass vaccination, antibiotic, and anti-malaria treatment campaigns.[166][171][172]Research on the timing of most recent common ancestor for HIV-1 groups M and O, as well as on HIV-2 groups A and B, indicates that SIV has given rise to transmissible HIV lineages throughout the twentieth century.[173]The dispersed timing of these transmissions to humans implies that no single external factor is needed to explain the cross-species transmission of HIV. This observation is consistent with both of the two prevailing views of the origin of the HIV epidemics, namely SIV transmission to humans during the slaughter or butchering of infected primates, and the colonial expansion of sub-Saharan African cities.[173]
See also
- Antiviral drug
- Discovery and development of HIV-protease inhibitors
- HIV/AIDS denialism
- HIVToolbox
- World AIDS Day
References
- ^Weiss RA (May 1993). "How does HIV cause AIDS?".Science.260(5112): 1273–9.Bibcode:1993Sci...260.1273W.doi:10.1126/science.8493571.PMID8493571.
- ^Douek DC, Roederer M, Koup RA (2009)."Emerging Concepts in the Immunopathogenesis of AIDS".Annual Review of Medicine.60:471–84.doi:10.1146/annurev.med.60.041807.123549.PMC2716400.PMID18947296.
- ^abPowell MK, Benková K, Selinger P, Dogoši M, Kinkorová Luňáčková I, Koutníková H, Laštíková J, Roubíčková A, Špůrková Z, Laclová L, Eis V, Šach J, Heneberg P (2016)."Opportunistic Infections in HIV-Infected Patients Differ Strongly in Frequencies and Spectra between Patients with Low CD4+ Cell Counts Examined Postmortem and Compensated Patients Examined Antemortem Irrespective of the HAART Era".PLOS ONE.11(9): e0162704.Bibcode:2016PLoSO..1162704P.doi:10.1371/journal.pone.0162704.PMC5017746.PMID27611681.
- ^UNAIDS, WHO (December 2007)."2007 AIDS epidemic update"(PDF).p. 16.
- ^abcdeRodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, Degen O, et al. (June 2019)."Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study".Lancet.393(10189): 2428–2438.doi:10.1016/S0140-6736(19)30418-0.PMC6584382.PMID31056293.
- ^abcEisinger RW, Dieffenbach CW,Fauci AS(February 2019). "HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable".JAMA.321(5): 451–452.doi:10.1001/jama.2018.21167.PMID30629090.S2CID58599661.
- ^Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J (2012). Desrosiers RC (ed.)."HIV-Specific Antibodies Capable of ADCC Are Common in Breastmilk and Are Associated with Reduced Risk of Transmission in Women with High Viral Loads".PLOS Pathogens.8(6): e1002739.doi:10.1371/journal.ppat.1002739.PMC3375288.PMID22719248.
- ^Hahn RA, Inhorn MC, eds. (2009).Anthropology and public health: bridging differences in culture and society(2nd ed.). Oxford: Oxford University Press. p. 449.ISBN978-0-19-537464-3.OCLC192042314.
- ^Mead MN (2008)."Contaminants in human milk: weighing the risks against the benefits of breastfeeding".Environmental Health Perspectives.116(10): A426–34.doi:10.1289/ehp.116-a426.PMC2569122.PMID18941560.Archived fromthe originalon 6 November 2008.
- ^
 This article incorporates text from this source, which is in thepublic domain:"Preventing Mother-to-Child Transmission of HIV".HIV.gov.May 15, 2017.RetrievedDecember 8,2017.
This article incorporates text from this source, which is in thepublic domain:"Preventing Mother-to-Child Transmission of HIV".HIV.gov.May 15, 2017.RetrievedDecember 8,2017.
- ^Cunningham AL, Donaghy H, Harman AN, Kim M, Turville SG (August 2010). "Manipulation of dendritic cell function by viruses".Current Opinion in Microbiology.13(4): 524–9.doi:10.1016/j.mib.2010.06.002.PMID20598938.
- ^Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, et al. (January 2014)."Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection".Nature.505(7484): 509–14.Bibcode:2014Natur.505..509D.doi:10.1038/nature12940.PMC4047036.PMID24356306.
- ^Garg H, Mohl J, Joshi A (November 2012)."HIV-1 induced bystander apoptosis".Viruses.4(11): 3020–43.doi:10.3390/v4113020.PMC3509682.PMID23202514.
- ^Kumar V (2012).Robbins Basic Pathology(9th ed.). Elsevier Health Sciences. p. 147.ISBN978-1-4557-3787-1.
- ^International Committee on Taxonomy of Viruses(2002)."61.0.6. Lentivirus".National Institutes of Health.Archived from the original on October 14, 2006.RetrievedFebruary 28,2006.
{{cite web}}:CS1 maint: unfit URL (link) - ^International Committee on Taxonomy of Viruses (2002)."61. Retroviridae".National Institutes of Health. Archived from the original on October 2, 2006.RetrievedFebruary 28,2006.
{{cite web}}:CS1 maint: unfit URL (link) - ^Levy JA (November 1993). "HIV pathogenesis and long-term survival".AIDS.7(11): 1401–10.doi:10.1097/00002030-199311000-00001.PMID8280406.
- ^Smith JA, Daniel R (May 2006). "Following the path of the virus: the exploitation of host DNA repair mechanisms by retroviruses".ACS Chemical Biology.1(4): 217–26.doi:10.1021/cb600131q.PMID17163676.
- ^abSiliciano RF, Greene WC (September 2011)."HIV latency".Cold Spring Harbor Perspectives in Medicine.1(1): a007096.doi:10.1101/cshperspect.a007096.PMC3234450.PMID22229121.
- ^Gilbert PB, McKeague IW, Eisen G, Mullins C, Guéye-NDiaye A, Mboup S, Kanki PJ (February 28, 2003). "Comparison of HIV-1 and HIV-2 infectivity from a prospective cohort study in Senegal".Statistics in Medicine.22(4): 573–593.doi:10.1002/sim.1342.PMID12590415.S2CID28523977.
- ^abReeves JD, Doms RW (2002)."Human Immunodeficiency Virus Type 2".Journal of General Virology.83(Pt 6): 1253–65.doi:10.1099/0022-1317-83-6-1253.PMID12029140.
- ^McGovern SL, Caselli E, Grigorieff N, Shoichet BK (2002). "A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening".Journal of Medicinal Chemistry.45(8): 1712–22.doi:10.1021/jm010533y.hdl:11380/977912.PMID11931626.
- ^Compared with overview in:Fisher B, Harvey RP, Champe PC (2007).Lippincott's Illustrated Reviews: Microbiology.Hagerstown, MD: Lippincott Williams & Wilkins. p. 3.ISBN978-0-7817-8215-9.
- ^abcdefgVarious (2008).HIV Sequence Compendium 2008 Introduction(PDF).RetrievedMarch 31,2009.
- ^abcChan DC, Fass D, Berger JM, Kim PS (April 1997)."Core structure of gp41 from the HIV envelope glycoprotein"(PDF).Cell.89(2): 263–73.doi:10.1016/S0092-8674(00)80205-6.PMID9108481.S2CID4518241.
- ^Klein JS, Bjorkman PJ (May 2010)."Few and far between: how HIV may be evading antibody avidity".PLOS Pathogens.6(5): e1000908.doi:10.1371/journal.ppat.1000908.PMC2877745.PMID20523901.
- ^National Institute of Health (June 17, 1998)."Crystal structure of key HIV protein reveals new prevention, treatment targets"(Press release). Archived fromthe originalon February 19, 2006.RetrievedSeptember 14,2006.
- ^Behrens AJ, Vasiljevic S, Pritchard LK, Harvey DJ, Andev RS, Krumm SA, et al. (March 2016)."Composition and Antigenic Effects of Individual Glycan Sites of a Trimeric HIV-1 Envelope Glycoprotein".Cell Reports.14(11): 2695–706.doi:10.1016/j.celrep.2016.02.058.PMC4805854.PMID26972002.
- ^Pritchard LK, Spencer DI, Royle L, Bonomelli C, Seabright GE, Behrens AJ, et al. (June 2015)."Glycan clustering stabilizes the mannose patch of HIV-1 and preserves vulnerability to broadly neutralizing antibodies".Nature Communications.6:7479.Bibcode:2015NatCo...6.7479P.doi:10.1038/ncomms8479.PMC4500839.PMID26105115.
- ^Pritchard LK, Harvey DJ, Bonomelli C, Crispin M, Doores KJ (September 2015)."Cell- and Protein-Directed Glycosylation of Native Cleaved HIV-1 Envelope".Journal of Virology.89(17): 8932–44.doi:10.1128/JVI.01190-15.PMC4524065.PMID26085151.
- ^Crispin M,Doores KJ(April 2015)."Targeting host-derived glycans on enveloped viruses for antibody-based vaccine design".Current Opinion in Virology.11:63–9.doi:10.1016/j.coviro.2015.02.002.PMC4827424.PMID25747313.
- ^Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, et al. (December 2013)."Crystal structure of a soluble cleaved HIV-1 envelope trimer".Science.342(6165): 1477–83.Bibcode:2013Sci...342.1477J.doi:10.1126/science.1245625.PMC3886632.PMID24179159.
- ^Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, et al. (December 2013)."Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer".Science.342(6165): 1484–90.Bibcode:2013Sci...342.1484L.doi:10.1126/science.1245627.PMC3954647.PMID24179160.
- ^Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, et al. (September 2013)."A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies".PLOS Pathogens.9(9): e1003618.doi:10.1371/journal.ppat.1003618.PMC3777863.PMID24068931.
- ^Pritchard LK, Vasiljevic S, Ozorowski G, Seabright GE, Cupo A, Ringe R, et al. (June 2015)."Structural Constraints Determine the Glycosylation of HIV-1 Envelope Trimers".Cell Reports.11(10): 1604–13.doi:10.1016/j.celrep.2015.05.017.PMC4555872.PMID26051934.
- ^de Taeye SW, Ozorowski G, Torrents de la Peña A, Guttman M, Julien JP, van den Kerkhof TL, et al. (December 2015)."Immunogenicity of Stabilized HIV-1 Envelope Trimers with Reduced Exposure of Non-neutralizing Epitopes".Cell.163(7): 1702–15.doi:10.1016/j.cell.2015.11.056.PMC4732737.PMID26687358.
- ^Ouellet DL, Plante I, Landry P, Barat C, Janelle ME, Flamand L, Tremblay MJ, Provost P (April 2008)."Identification of functional microRNAs released through asymmetrical processing of HIV-1 TAR element".Nucleic Acids Research.36(7): 2353–65.doi:10.1093/nar/gkn076.PMC2367715.PMID18299284.
- ^Klase Z, Winograd R, Davis J, Carpio L, Hildreth R, Heydarian M, Fu S, McCaffrey T, Meiri E, Ayash-Rashkovsky M, Gilad S, Bentwich Z, Kashanchi F (2009)."HIV-1 TAR miRNA protects against apoptosis by altering cellular gene expression".Retrovirology.6(1): 18.doi:10.1186/1742-4690-6-18.PMC2654423.PMID19220914.
- ^Vasudevan AA, Smits SH, Höppner A, Häussinger D, Koenig BW, Münk C (November 2013)."Structural features of antiviral DNA cytidine deaminases".Biological Chemistry(Submitted manuscript).394(11): 1357–70.doi:10.1515/hsz-2013-0165.PMID23787464.S2CID4151961.
- ^Garcia JV, Miller AD (April 1991). "Serine phosphorylation-independent downregulation of cell-surface CD4 by nef".Nature.350(6318): 508–11.Bibcode:1991Natur.350..508G.doi:10.1038/350508a0.PMID2014052.S2CID1628392.
- ^Schwartz O, Maréchal V, Le Gall S, Lemonnier F, Heard JM (March 1996). "Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein".Nature Medicine.2(3): 338–42.doi:10.1038/nm0396-338.PMID8612235.S2CID7461342.
- ^Stumptner-Cuvelette P, Morchoisne S, Dugast M, Le Gall S, Raposo G, Schwartz O, Benaroch P (October 2001)."HIV-1 Nef impairs MHC class II antigen presentation and surface expression".Proceedings of the National Academy of Sciences of the United States of America.98(21): 12144–9.Bibcode:2001PNAS...9812144S.doi:10.1073/pnas.221256498.PMC59782.PMID11593029.
- ^Arrildt KT, Joseph SB, Swanstrom R (March 2012)."The HIV-1 env protein: a coat of many colors".Current HIV/AIDS Reports.9(1): 52–63.doi:10.1007/s11904-011-0107-3.PMC3658113.PMID22237899.
- ^abBerger EA, Doms RW, Fenyö EM, Korber BT, Littman DR, Moore JP, Sattentau QJ, Schuitemaker H, Sodroski J, Weiss RA (1998)."A new classification for HIV-1".Nature.391(6664): 240.Bibcode:1998Natur.391..240B.doi:10.1038/34571.PMID9440686.S2CID2159146.
- ^abcCoakley E, Petropoulos CJ, Whitcomb JM (2005). "Assessing ch vbgemokine co-receptor usage in HIV".Current Opinion in Infectious Diseases.18(1): 9–15.doi:10.1097/00001432-200502000-00003.PMID15647694.S2CID30923492.
- ^ Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR (1996). "Identification of a major co-receptor for primary isolates of HIV-1".Nature.381(6584): 661–6.Bibcode:1996Natur.381..661D.doi:10.1038/381661a0.PMID8649511.S2CID37973935.
- ^ Feng Y, Broder CC, Kennedy PE, Berger EA (1996)."HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor".Science.272(5263): 872–7.Bibcode:1996Sci...272..872F.doi:10.1126/science.272.5263.872.PMC3412311.PMID8629022.S2CID44455027.
- ^Knight SC, Macatonia SE, Patterson S (1990). "HIV I infection of dendritic cells".International Review of Immunology.6(2–3): 163–75.doi:10.3109/08830189009056627.PMID2152500.
- ^Tang J, Kaslow RA (2003)."The impact of host genetics on HIV infection and disease progression in the era of highly active antiretroviral therapy".AIDS.17(Suppl 4): S51–S60.doi:10.1097/00002030-200317004-00006.PMID15080180.
- ^Zhu T, Mo H, Wang N, Nam DS, Cao Y, Koup RA, Ho DD (1993). "Genotypic and phenotypic characterization of HIV-1 patients with primary infection".Science.261(5125): 1179–81.Bibcode:1993Sci...261.1179Z.doi:10.1126/science.8356453.PMID8356453.
- ^van't Wout AB, Kootstra NA, Mulder-Kampinga GA, Albrecht-van Lent N, Scherpbier HJ, Veenstra J, Boer K, Coutinho RA, Miedema F, Schuitemaker H (1994)."Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission".Journal of Clinical Investigation.94(5): 2060–7.doi:10.1172/JCI117560.PMC294642.PMID7962552.
- ^Zhu T, Wang N, Carr A, Nam DS, Moor-Jankowski R, Cooper DA, Ho DD (1996)."Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission".Journal of Virology.70(5): 3098–107.doi:10.1128/JVI.70.5.3098-3107.1996.PMC190172.PMID8627789.
- ^Clevestig P, Maljkovic I, Casper C, Carlenor E, Lindgren S, Navér L, Bohlin AB, Fenyö EM, Leitner T, Ehrnst A (2005). "The X4 phenotype of HIV type 1 evolves from R5 in two children of mothers, carrying X4, and is not linked to transmission".AIDS Research and Human Retroviruses.21(5): 371–8.doi:10.1089/aid.2005.21.371.PMID15929699.
- ^Moore JP (1997). "Coreceptors: implications for HIV pathogenesis and therapy".Science.276(5309): 51–2.doi:10.1126/science.276.5309.51.PMID9122710.S2CID33262844.
- ^Karlsson A, Parsmyr K, Aperia K, Sandström E, Fenyö EM, Albert J (1994). "MT-2 cell tropism of human immunodeficiency virus type 1 isolates as a marker for response to treatment and development of drug resistance".The Journal of Infectious Diseases.170(6): 1367–75.doi:10.1093/infdis/170.6.1367.PMID7995974.
- ^Koot M, van 't Wout AB, Kootstra NA, de Goede RE, Tersmette M, Schuitemaker H (1996)."Relation between changes in cellular load, evolution of viral phenotype, and the clonal composition of virus populations in the course of human immunodeficiency virus type 1 infection".The Journal of Infectious Diseases.173(2): 349–54.doi:10.1093/infdis/173.2.349.PMID8568295.
- ^Cheney K, McKnight A (2010). "HIV-2 Tropism and Disease".Lentiviruses and Macrophages: Molecular and Cellular Interactions.Caister Academic Press.ISBN978-1-904455-60-8.[page needed]
- ^abcdefgChan DC, Kim PS (1998)."HIV entry and its inhibition".Cell.93(5): 681–4.doi:10.1016/S0092-8674(00)81430-0.PMID9630213.S2CID10544941.
- ^abcdefWyatt R, Sodroski J (1998). "The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens".Science.280(5371): 1884–8.Bibcode:1998Sci...280.1884W.doi:10.1126/science.280.5371.1884.PMID9632381.
- ^abArthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, McLaughlin S, Pascuccio M, Gopaul R, McNally J, Cruz CC, Censoplano N, Chung E, Reitano KN, Kottilil S, Goode DJ, Fauci AS (2008). "HIV-1 envelope protein binds to and signals through integrin alpha(4)beta(7), the gut mucosal homing receptor for peripheral T cells".Nature Immunology.9(3): 301–9.doi:10.1038/ni1566.PMID18264102.S2CID205361178.
- ^abPope M, Haase AT (2003)."Transmission, acute HIV-1 infection and the quest for strategies to prevent infection".Nature Medicine.9(7): 847–52.doi:10.1038/nm0703-847.PMID12835704.S2CID26570505.
- ^Haedicke J, Brown C, Naghavi MH (August 2009)."The brain-specific factor FEZ1 is a determinant of neuronal susceptibility to HIV-1 infection".Proceedings of the National Academy of Sciences.106(33): 14040–14045.Bibcode:2009PNAS..10614040H.doi:10.1073/pnas.0900502106.PMC2729016.PMID19667186.
- ^Daecke J, Fackler OT, Dittmar MT, Kräusslich HG (2005)."Involvement of clathrin-mediated endocytosis in human immunodeficiency virus type 1 entry".Journal of Virology.79(3): 1581–1594.doi:10.1128/jvi.79.3.1581-1594.2005.PMC544101.PMID15650184.
- ^Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB (2009)."HIV Enters Cells via Endocytosis and Dynamin-Dependent Fusion with Endosomes".Cell.137(3): 433–444.doi:10.1016/j.cell.2009.02.046.PMC2696170.PMID19410541.
- ^Koch P, Lampe M, Godinez WJ, Müller B, Rohr K, Kräusslich HG, Lehmann MJ (2009)."Visualizing fusion of pseudotyped HIV-1 particles in real time by live cell microscopy".Retrovirology.6:84.doi:10.1186/1742-4690-6-84.PMC2762461.PMID19765276.
- ^Thorley JA, McKeating JA, Rappoport JZ (2010)."Mechanisms of viral entry: sneaking in the front door".Protoplasma.244(1–4): 15–24.doi:10.1007/s00709-010-0152-6.PMC3038234.PMID20446005.
- ^Permanyer M, Ballana E, Esté JA (2010). "Endocytosis of HIV: anything goes".Trends in Microbiology.18(12): 543–551.doi:10.1016/j.tim.2010.09.003.PMID20965729.
- ^abcZheng YH, Lovsin N, Peterlin BM (2005). "Newly identified host factors modulate HIV replication".Immunology Letters.97(2): 225–34.doi:10.1016/j.imlet.2004.11.026.PMID15752562.
- ^"IV. Viruses> F. Animal Virus Life Cycles > 3. The Life Cycle of HIV".Doc Kaiser's Microbiology Home Page.Community College of Baltimore County. January 2008. Archived fromthe originalon July 26, 2010.
- ^Hiscott J, Kwon H, Génin P (2001)."Hostile takeovers: viral appropriation of the NF-kB pathway".Journal of Clinical Investigation.107(2): 143–151.doi:10.1172/JCI11918.PMC199181.PMID11160127.
- ^Keane SC, Heng X, Lu K, Kharytonchyk S, Ramakrishnan V, Carter G, Barton S, Hosic A, Florwick A, Santos J, Bolden NC (May 22, 2015)."Structure of the HIV-1 RNA packaging signal".Science.348(6237): 917–921.Bibcode:2015Sci...348..917K.doi:10.1126/science.aaa9266.ISSN0036-8075.PMC4492308.PMID25999508.
- ^Keane SC, Van V, Frank HM, Sciandra CA, McCowin S, Santos J, Heng X, Summers MF (October 10, 2016)."NMR detection of intermolecular interaction sites in the dimeric 5′-leader of the HIV-1 genome".Proceedings of the National Academy of Sciences.113(46): 13033–13038.Bibcode:2016PNAS..11313033K.doi:10.1073/pnas.1614785113.ISSN0027-8424.PMC5135362.PMID27791166.
- ^Ocwieja KE, Sherrill-Mix S, Mukherjee R, Custers-Allen R, David P, Brown M, et al. (November 2012)."Dynamic regulation of HIV-1 mRNA populations analyzed by single-molecule enrichment and long-read sequencing".Nucleic Acids Research.40(20): 10345–55.doi:10.1093/nar/gks753.PMC3488221.PMID22923523.
- ^Pollard VW, Malim MH (1998). "The HIV-1 Rev protein".Annual Review of Microbiology.52:491–532.doi:10.1146/annurev.micro.52.1.491.PMID9891806.
- ^Butsch M, Boris-Lawrie K (April 2002)."Destiny of unspliced retroviral RNA: ribosome and/or virion?".Journal of Virology.76(7): 3089–94.doi:10.1128/JVI.76.7.3089-3094.2002.PMC136024.PMID11884533.
- ^Hellmund C, Lever AM (July 2016)."Coordination of Genomic RNA Packaging with Viral Assembly in HIV-1".Viruses.8(7): 192.doi:10.3390/v8070192.PMC4974527.PMID27428992.
- ^Soto-Rifo R, Limousin T, Rubilar PS, Ricci EP, Décimo D, Moncorgé O, et al. (March 2012)."Different effects of the TAR structure on HIV-1 and HIV-2 genomic RNA translation".Nucleic Acids Research.40(6): 2653–67.doi:10.1093/nar/gkr1093.PMC3315320.PMID22121214.
- ^Saad JS, Muriaux DM (July 28, 2015).Role of Lipids in Virus Assembly.Frontiers Media SA.ISBN978-2-88919-582-4.
- ^Ricci EP, Herbreteau CH, Decimo D, Schaupp A, Datta SA, Rein A, et al. (July 2008)."In vitro expression of the HIV-2 genomic RNA is controlled by three distinct internal ribosome entry segments that are regulated by the HIV protease and the Gag polyprotein".RNA.14(7): 1443–55.doi:10.1261/rna.813608.PMC2441975.PMID18495939.
- ^abHu WS, Temin HM (1990). "Retroviral recombination and reverse transcription".Science.250(4985): 1227–33.Bibcode:1990Sci...250.1227H.doi:10.1126/science.1700865.PMID1700865.
- ^abCharpentier C, Nora T, Tenaillon O, Clavel F, Hance AJ (2006)."Extensive recombination among human immunodeficiency virus type 1 quasispecies makes an important contribution to viral diversity in individual patients".Journal of Virology.80(5): 2472–82.doi:10.1128/JVI.80.5.2472-2482.2006.PMC1395372.PMID16474154.
- ^Nora T, Charpentier C, Tenaillon O, Hoede C, Clavel F, Hance AJ (2007)."Contribution of recombination to the evolution of human immunodeficiency viruses expressing resistance to antiretroviral treatment".Journal of Virology.81(14): 7620–8.doi:10.1128/JVI.00083-07.PMC1933369.PMID17494080.
- ^Chen J, Powell D, Hu WS (2006)."High frequency of genetic recombination is a common feature of primate lentivirus replication".Journal of Virology.80(19): 9651–8.doi:10.1128/JVI.00936-06.PMC1617242.PMID16973569.
- ^abBonhoeffer S, Chappey C, Parkin NT, Whitcomb JM, Petropoulos CJ (2004). "Evidence for positive epistasis in HIV-1".Science.306(5701): 1547–50.Bibcode:2004Sci...306.1547B.doi:10.1126/science.1101786.PMID15567861.S2CID45784964.
- ^Israël N, Gougerot-Pocidalo MA (1997)."Oxidative stress in human immunodeficiency virus infection".Cellular and Molecular Life Sciences.53(11–12): 864–70.doi:10.1007/s000180050106.PMC11147326.PMID9447238.S2CID22663454.
- ^Michod RE, Bernstein H, Nedelcu AM (May 2008)."Adaptive value of sex in microbial pathogens"(PDF).Infection, Genetics and Evolution.8(3): 267–85.Bibcode:2008InfGE...8..267M.doi:10.1016/j.meegid.2008.01.002.PMID18295550.
- ^Hallenberger S, Bosch V, Angliker H, Shaw E, Klenk HD, Garten W (November 26, 1992). "Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160".Nature.360(6402): 358–61.Bibcode:1992Natur.360..358H.doi:10.1038/360358a0.PMID1360148.S2CID4306605.
- ^Gelderblom HR (1997)."Fine structure of HIV and SIV"(PDF).In Los Alamos National Laboratory (ed.).HIV sequence compendium.Los Alamos National Laboratory.pp. 31–44.
- ^abcdZhang C, Zhou S, Groppelli E, Pellegrino P, Williams I, Borrow P, Chain BM, Jolly C (2015)."Hybrid Spreading Mechanisms and T Cell Activation Shape the Dynamics of HIV-1 Infection".PLOS Computational Biology.11(4): e1004179.arXiv:1503.08992.Bibcode:2015PLSCB..11E4179Z.doi:10.1371/journal.pcbi.1004179.PMC4383537.PMID25837979.
- ^abJolly C, Kashefi K, Hollinshead M, Sattentau QJ (2004)."HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse".Journal of Experimental Medicine.199(2): 283–293.doi:10.1084/jem.20030648.PMC2211771.PMID14734528.
- ^Sattentau Q (2008)."Avoiding the void: cell-to-cell spread of human viruses".Nature Reviews Microbiology.6(11): 815–826.doi:10.1038/nrmicro1972.PMID18923409.S2CID20991705.
- ^Duncan CJ, Russell RA, Sattentau QJ (2013)."High multiplicity HIV-1 cell-to-cell transmission from macrophages to CD4+ T cells limits antiretroviral efficacy".AIDS.27(14): 2201–2206.doi:10.1097/QAD.0b013e3283632ec4.PMC4714465.PMID24005480.
- ^Sewald X, Gonzalez DG, Haberman AM, Mothes W (2012)."In vivo imaging of virological synapses".Nature Communications.3:1320.Bibcode:2012NatCo...3.1320S.doi:10.1038/ncomms2338.PMC3784984.PMID23271654.
- ^Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, Baltimore D (2011)."Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy".Nature.477(7362): 95–98.Bibcode:2011Natur.477...95S.doi:10.1038/nature10347.PMID21849975.S2CID4409389.
- ^abcdRobertson DL, Hahn BH, Sharp PM (1995)."Recombination in AIDS viruses".Journal of Molecular Evolution.40(3): 249–59.Bibcode:1995JMolE..40..249R.doi:10.1007/BF00163230.PMID7723052.S2CID19728830.
- ^Rambaut A, Posada D, Crandall KA, Holmes EC (January 2004)."The causes and consequences of HIV evolution".Nature Reviews Genetics.5(52–61): 52–61.doi:10.1038/nrg1246.PMID14708016.S2CID5790569.
- ^Perelson AS, Ribeiro RM (October 2008)."Estimating drug efficacy and viral dynamic parameters: HIV and HCV".Statistics in Medicine.27(23): 4647–57.doi:10.1002/sim.3116.PMID17960579.S2CID33662579.
- ^abSodora DL, Allan JS, Apetrei C, Brenchley JM, Douek DC, Else JG, Estes JD, Hahn BH, Hirsch VM, Kaur A, Kirchhoff F, Muller-Trutwin M, Pandrea I, Schmitz JE, Silvestri G (2009)."Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts".Nature Medicine.15(8): 861–865.doi:10.1038/nm.2013.PMC2782707.PMID19661993.
- ^Holzammer S, Holznagel E, Kaul A, Kurth R, Norley S (2001)."High virus loads in naturally and experimentally SIVagm-infected African green monkeys".Virology.283(2): 324–31.doi:10.1006/viro.2001.0870.PMID11336557.
- ^Kurth R, Norley S (1996). "Why don't the natural hosts of SIV develop simian AIDS?".The Journal of NIH Research.8:33–37.
- ^Baier M, Dittmar MT, Cichutek K, Kurth R (1991)."Development of vivo of genetic variability of simian immunodeficiency virus".Proceedings of the National Academy of Sciences of the United States of America.88(18): 8126–30.Bibcode:1991PNAS...88.8126B.doi:10.1073/pnas.88.18.8126.PMC52459.PMID1896460.
- ^Daniel MD, King NW, Letvin NL, Hunt RD, Sehgal PK, Desrosiers RC (1984). "A new type D retrovirus isolated from macaques with an immunodeficiency syndrome".Science.223(4636): 602–5.Bibcode:1984Sci...223..602D.doi:10.1126/science.6695172.PMID6695172.
- ^abKeele BF, Jones JH, Terio KA, Estes JD, Rudicell RS, Wilson ML, Li Y, Learn GH, Beasley TM, Schumacher-Stankey J, Wroblewski E, Mosser A, Raphael J, Kamenya S, Lonsdorf EV, Travis DA, Mlengeya T, Kinsel MJ, Else JG, Silvestri G, Goodall J, Sharp PM, Shaw GM, Pusey AE, Hahn BH (2009)."Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz".Nature.460(7254): 515–519.Bibcode:2009Natur.460..515K.doi:10.1038/nature08200.PMC2872475.PMID19626114.
- ^Schindler M, Münch J, Kutsch O, Li H, Santiago ML, Bibollet-Ruche F, Müller-Trutwin MC, Novembre FJ, Peeters M, Courgnaud V, Bailes E, Roques P, Sodora DL, Silvestri G, Sharp PM, Hahn BH, Kirchhoff F (2006)."Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1".Cell.125(6): 1055–67.doi:10.1016/j.cell.2006.04.033.PMID16777597.S2CID15132918.
- ^Thomson MM, Pérez-Alvarez L, Nájera R (2002). "Molecular epidemiology of HIV-1 genetic forms and its significance for vaccine development and therapy".The Lancet Infectious Diseases.2(8): 461–471.doi:10.1016/S1473-3099(02)00343-2.PMID12150845.
- ^Carr JK, Foley BT, Leitner T, Salminen M, Korber B, McCutchan F (1998)."Reference sequences representing the principal genetic diversity of HIV-1 in the pandemic"(PDF).In Los Alamos National Laboratory (ed.).HIV sequence compendium.Los Alamos, New Mexico:Los Alamos National Laboratory.pp. 10–19.
- ^Osmanov S, Pattou C, Walker N, Schwardländer B, Esparza J (2002). "Estimated global distribution and regional spread of HIV-1 genetic subtypes in the year 2000".Journal of Acquired Immune Deficiency Syndromes.29(2): 184–190.doi:10.1097/00042560-200202010-00013.PMID11832690.S2CID12536801.
- ^Perrin L, Kaiser L, Yerly S (2003). "Travel and the spread of HIV-1 genetic variants".The Lancet Infectious Diseases.3(1): 22–27.doi:10.1016/S1473-3099(03)00484-5.PMID12505029.
- ^abPlantier JC, Leoz M, Dickerson JE, De Oliveira F, Cordonnier F, Lemée V, Damond F, Robertson DL, Simon F (August 2009). "A new human immunodeficiency virus derived from gorillas".Nature Medicine.15(8): 871–2.doi:10.1038/nm.2016.PMID19648927.S2CID76837833.
- ^Smith L (August 3, 2009)."Woman found carrying new strain of HIV from gorillas".The Independent.RetrievedNovember 27,2015.
- ^Sharp PM, Hahn BH (August 2010)."The evolution of HIV-1 and the origin of AIDS".Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.365(1552): 2487–94.doi:10.1098/rstb.2010.0031.PMC2935100.PMID20643738.
- ^Keele BF, Van Heuverswyn F, Li Y, Bailes E, Takehisa J, Santiago ML, et al. (July 2006)."Chimpanzee reservoirs of pandemic and nonpandemic HIV-1".Science.313(5786): 523–6.Bibcode:2006Sci...313..523K.doi:10.1126/science.1126531.PMC2442710.PMID16728595.
- ^abcd Kumaranayake L, Watts C (2001). "Resource allocation and priority setting of HIV/AIDS interventions: addressing the generalized epidemic in sub-Saharan Africa".Journal of International Development.13(4): 451–466.doi:10.1002/jid.797.
- ^Kleinman S (September 2004)."Patient information: Blood donation and transfusion".Uptodate. Archived fromthe originalon April 12, 2008.
- ^abCenters for Disease Control and Prevention (2001). "Revised guidelines for HIV counseling, testing, and referral".MMWR Recommendations and Reports.50(RR–19): 1–57.PMID11718472.
- ^Celum CL, Coombs RW, Lafferty W, Inui TS, Louie PH, Gates CA, McCreedy BJ, Egan R, Grove T, Alexander S (1991). "Indeterminate human immunodeficiency virus type 1 western blots: seroconversion risk, specificity of supplemental tests, and an algorithm for evaluation".The Journal of Infectious Diseases.164(4): 656–664.doi:10.1093/infdis/164.4.656.PMID1894929.
- ^"Country Comparison:: HIV/AIDS - Deaths".The World Factbook, Central Intelligence Agency. Archived fromthe originalon April 30, 2017.RetrievedNovember 22,2015.
- ^Chou R, Selph S, Dana T, Bougatsos C, Zakher B, Blazina I, Korthuis PT (November 2012)."Screening for HIV: systematic review to update the 2005 U.S. Preventive Services Task Force recommendation".Annals of Internal Medicine.157(10): 706–18.doi:10.7326/0003-4819-157-10-201211200-00007.PMID23165662.S2CID27494096.
- ^Chou R, Huffman LH, Fu R, Smits AK, Korthuis PT (July 2005)."Screening for HIV: a review of the evidence for the U.S. Preventive Services Task Force".Annals of Internal Medicine.143(1): 55–73.doi:10.7326/0003-4819-143-1-200507050-00010.PMID15998755.S2CID24086322.
- ^Tolle MA, Schwarzwald HL (July 2010)."Postexposure prophylaxis against human immunodeficiency virus".American Family Physician.82(2): 161–6.PMID20642270.Archivedfrom the original on November 28, 2023.
- ^"Quick Reference Guide—Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations"(PDF).Centers for Disease Control and Prevention.New York State Department of Health. June 27, 2014. pp. 1–2. Archived fromthe original(PDF)on March 2, 2017.RetrievedApril 13,2017.
- ^"HIV Treatment: FDA-Approved HIV Medicines".AIDSinfo. Archived fromthe originalon February 23, 2017.RetrievedOctober 7,2016.
- ^Rodari A, Darcis G, Van Lint CM (September 29, 2021)."The Current Status of Latency Reversing Agents for HIV-1 Remission".Annual Review of Virology.8(1): 491–514.doi:10.1146/annurev-virology-091919-103029.ISSN2327-056X.PMID34586875.
- ^Swiss National AIDS Commission (October 15, 2016)."The Swiss statement".HIV i-Base.RetrievedApril 2,2019.
- ^Vernazza P, Bernard EJ (January 29, 2016)."HIV is not transmitted under fully suppressive therapy: The Swiss Statement—eight years later".Swiss Medical Weekly.146:w14246.doi:10.4414/smw.2016.14246.PMID26824882.
- ^The Lancet HIV (November 2017)."U=U taking off in 2017".Editorial.The Lancet. HIV.4(11): e475.doi:10.1016/S2352-3018(17)30183-2.PMID29096785.
- ^"Can't Pass It On".Terrence Higgins Trust.2019.Archivedfrom the original on April 7, 2019.RetrievedApril 2,2019.
- ^Bavinton BR, Pinto AN, Phanuphak N, Grinsztejn B, Prestage GP, Zablotska-Manos IB, et al. (August 2018). "Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study".The Lancet. HIV.5(8): e438–e447.doi:10.1016/S2352-3018(18)30132-2.PMID30025681.S2CID51702998.
- ^Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. (July 2016)."Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy".JAMA.316(2): 171–81.doi:10.1001/jama.2016.5148.PMID27404185.
- ^Rodger A( (July 2018).Risk of HIV transmission through condomless sex in MSM couples with suppressive ART: The PARTNER2 Study extended results in gay men.AIDS2018: 22nd International AIDS Conference. Amsterdam, the Netherlands.RetrievedApril 2,2019.
- ^abcHoffman H (January 10, 2019)."The science is clear: with HIV, undetectable equals untransmittable"(Press release).National Institutes of Health.National Institute of Allergy and Infectious Diseases.RetrievedMay 3,2019.
NIAID Director Anthony S. Fauci, M.D., and colleagues summarize results from large clinical trials and cohort studies validating U=U. The landmark NIH-funded HPTN 052 clinical trial showed that no linked HIV transmissions occurred among HIV serodifferent heterosexual couples when the partner living with HIV had a durably suppressed viral load. Subsequently, the PARTNER and Opposites Attract studies confirmed these findings and extended them to male-male couples.... The success of U=U as an HIV prevention method depends on achieving and maintaining an undetectable viral load by taking ART daily as prescribed.
- ^Cohen MS,Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. (September 2016)."Antiretroviral Therapy for the Prevention of HIV-1 Transmission".The New England Journal of Medicine.375(9): 830–9.doi:10.1056/NEJMoa1600693.PMC5049503.PMID27424812.
- ^Hodson M (November 17, 2017).U=U: Talking to patients about transmission risk(PDF).British HIV Association Autumn Conference 2017.RetrievedMay 3,2019.(abstractfor presentation on behalf ofNAM / Aidsmap)
- ^"Consensus statement: Risk of Sexual Transmission of HIV from a Person Living with HIV who has an Undetectable Viral Load".Prevention Access Campaign.July 21, 2016.RetrievedApril 2,2019.Note:When the statement and list of endorsements was retrieved, it had last been updated on 23 August 2018 and included "over 850 organizations from nearly 100 countries."
- ^Boseley S,Devlin H(May 3, 2019)."End to AIDS in sight as huge study finds drugs stop HIV transmission".The Guardian.RetrievedMay 3,2019.
- ^Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, et al. (August 2009)."Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition".Nature Medicine.15(8): 886–92.doi:10.1038/nm.2006.PMC2723183.PMID19648930.
- ^Looker KJ, Elmes JA, Gottlieb SL, Schiffer JT, Vickerman P, Turner KM, Boily MC (December 2017)."Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis".The Lancet. Infectious Diseases.17(12): 1303–1316.doi:10.1016/S1473-3099(17)30405-X.PMC5700807.PMID28843576.
- ^"On this day".News & Record.May 18, 2020. pp. 2A.
- ^Mandell GL, Bennett JE, Dolin R, eds. (2010). "Chapter 169".Mandell, Douglas, and Bennett's principles and practice of infectious diseases(7th ed.). Philadelphia: Churchill Livingstone/Elsevier.ISBN978-0-443-06839-3.[page needed]
- ^Gottlieb MS (2006)."Pneumocystis pneumonia—Los Angeles. 1981".American Journal of Public Health.96(6): 980–1, discussion 982–3.doi:10.2105/AJPH.96.6.980.PMC1470612.PMID16714472.Archivedfrom the original on April 22, 2009.
- ^Friedman-Kien AE (October 1981). "Disseminated Kaposi's sarcoma syndrome in young homosexual men".Journal of the American Academy of Dermatology.5(4): 468–71.doi:10.1016/S0190-9622(81)80010-2.PMID7287964.
- ^Hymes KB, Cheung T, Greene JB, Prose NS, Marcus A, Ballard H, William DC, Laubenstein LJ (September 1981). "Kaposi's sarcoma in homosexual men — a report of eight cases".The Lancet.2(8247): 598–600.doi:10.1016/S0140-6736(81)92740-9.PMID6116083.S2CID43529542.
- ^abBasavapathruni A, Anderson KS (December 2007)."Reverse transcription of the HIV-1 pandemic".The FASEB Journal.21(14): 3795–3808.doi:10.1096/fj.07-8697rev.PMID17639073.S2CID24960391.
- ^Lederberg J, ed. (2000).Encyclopedia of Microbiology(2nd ed.). Burlington: Elsevier. p. 106.ISBN978-0-08-054848-7.RetrievedJune 9,2016.
- ^Centers for Disease Control (1982)."Persistent, generalized lymphadenopathy among homosexual males".Morbidity and Mortality Weekly Report.31(19): 249–251.PMID6808340.
- ^abBarré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L (1983). "Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS)".Science.220(4599): 868–871.Bibcode:1983Sci...220..868B.doi:10.1126/science.6189183.PMID6189183.S2CID390173.
- ^abCenters for Disease Control (1982)."Opportunistic infections and Kaposi's sarcoma among Haitians in the United States".Morbidity and Mortality Weekly Report.31(26): 353–354, 360–361.PMID6811853.
- ^Altman LK (May 11, 1982)."New homosexual disorder worries health officials".The New York Times.RetrievedAugust 31,2011.
- ^Gilman SL (1987). GilmanSL (ed.). "AIDS and Syphilis: The Iconography of Disease".October.43:87–107.doi:10.2307/3397566.JSTOR3397566.
- ^"Making Headway Under Hellacious Circumstances"(PDF).American Association for the Advancement of Science.July 28, 2006. Archived fromthe original(PDF)on June 24, 2008.RetrievedJune 23,2008.
- ^Kher U (July 27, 1982)."A Name for the Plague".Time.Archived fromthe originalon March 7, 2008.RetrievedMarch 10,2008.
- ^Centers for Disease Control (1982). "Update on acquired immune deficiency syndrome (AIDS)—United States".Morbidity and Mortality Weekly Report.31(37): 507–508, 513–514.PMID6815471.
- ^Gallo RC, Sarin PS, Gelmann EP, Robert-Guroff M, Richardson E, Kalyanaraman VS, Mann D, Sidhu GD, Stahl RE, Zolla-Pazner S, Leibowitch J, Popovic M (1983). "Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS)".Science.220(4599): 865–867.Bibcode:1983Sci...220..865G.doi:10.1126/science.6601823.PMID6601823.
- ^"The 2008 Nobel Prize in Physiology or Medicine - Press Release".www.nobelprize.org.RetrievedJanuary 28,2018.
- ^Crewdson J (May 30, 1991)."GALLO ADMITS FRENCH DISCOVERED AIDS VIRUS".Chicago Tribune.RetrievedApril 25,2020.
- ^Aldrich R, Wotherspoon G, eds. (2001).Who's who in gay and lesbian history.London: Routledge. p. 154.ISBN978-0-415-22974-6.
- ^Levy JA, et al. (1984). "Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS".Science.225(4664): 840–842.Bibcode:1984Sci...225..840L.doi:10.1126/science.6206563.PMID6206563.
- ^Levy JA, Kaminsky LS, Morrow WJ, Steimer K, Luciw P, Dina D, Hoxie J, Oshiro L (1985). "Infection by the retrovirus associated with the acquired immunodeficiency syndrome".Annals of Internal Medicine.103(5): 694–699.doi:10.7326/0003-4819-103-5-694.PMID2996401.
- ^Sharp PM, Hahn BH (2011)."Origins of HIV and the AIDS Pandemic".Cold Spring Harbor Perspectives in Medicine.1(1): a006841.doi:10.1101/cshperspect.a006841.PMC3234451.PMID22229120.
- ^Faria NR, Rambaut A, Suchard MA, Baele G, Bedford T, Ward MJ, Tatem AJ, Sousa JD, Arinaminpathy N, Pépin J, Posada D, Peeters M, Pybus OG, Lemey P (2014)."The early spread and epidemic ignition of HIV-1 in human populations".Science.346(6205): 56–61.Bibcode:2014Sci...346...56F.doi:10.1126/science.1256739.PMC4254776.PMID25278604.
- ^Gao F, Bailes E, Robertson DL, Chen Y, Rodenburg CM, Michael SF, Cummins LB, Arthur LO, Peeters M, Shaw GM, Sharp PM, Hahn BH (1999)."Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes".Nature.397(6718): 436–41.Bibcode:1999Natur.397..436G.doi:10.1038/17130.PMID9989410.S2CID4432185.
- ^Keele BF, Van Heuverswyn F, Li Y, Bailes E, Takehisa J, Santiago ML, Bibollet-Ruche F, Chen Y, Wain LV, Liegeois F, Loul S, Ngole EM, Bienvenue Y, Delaporte E, Brookfield JF, Sharp PM, Shaw GM, Peeters M, Hahn BH (2006)."Chimpanzee reservoirs of pandemic and nonpandemic HIV-1".Science.313(5786): 523–6.Bibcode:2006Sci...313..523K.doi:10.1126/science.1126531.PMC2442710.PMID16728595.
- ^Goodier JL, Kazazian HH (2008)."Retrotransposons revisited: the restraint and rehabilitation of parasites".Cell.135(1): 23–35.doi:10.1016/j.cell.2008.09.022.PMID18854152.S2CID3093360.
- ^Sharp PM, Bailes E, Chaudhuri RR, Rodenburg CM, Santiago MO, Hahn BH (2001)."The origins of acquired immune deficiency syndrome viruses: where and when?".Philosophical Transactions of the Royal Society B.356(1410): 867–76.doi:10.1098/rstb.2001.0863.PMC1088480.PMID11405934.
- ^Kalish ML, Wolfe ND, Ndongmo CB, McNicholl J, Robbins KE, Aidoo M, Fonjungo PN, Alemnji G, Zeh C, Djoko CF, Mpoudi-Ngole E, Burke DS, Folks TM (2005)."Central African hunters exposed to simian immunodeficiency virus".Emerging Infectious Diseases.11(12): 1928–30.doi:10.3201/eid1112.050394.PMC3367631.PMID16485481.
- ^abMarx PA, Alcabes PG, Drucker E (2001)."Serial human passage of simian immunodeficiency virus by unsterile injections and the emergence of epidemic human immunodeficiency virus in Africa".Philosophical Transactions of the Royal Society B.356(1410): 911–20.doi:10.1098/rstb.2001.0867.PMC1088484.PMID11405938.
- ^Worobey M, Gemmel M, Teuwen DE, Haselkorn T, Kunstman K, Bunce M, Muyembe JJ, Kabongo JM, Kalengayi RM, Van Marck E, Gilbert MT, Wolinsky SM (2008)."Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960".Nature.455(7213): 661–4.Bibcode:2008Natur.455..661W.doi:10.1038/nature07390.PMC3682493.PMID18833279.
- ^abde Sousa JD, Müller V, Lemey P, Vandamme AM (2010). Martin DP (ed.)."High GUD incidence in the early 20th century created a particularly permissive time window for the origin and initial spread of epidemic HIV strains".PLOS ONE.5(4): e9936.Bibcode:2010PLoSO...5.9936S.doi:10.1371/journal.pone.0009936.PMC2848574.PMID20376191.
- ^Zhu T, Korber BT, Nahmias AJ, Hooper E, Sharp PM, Ho DD (1998)."An African HIV-1 Sequence from 1959 and Implications for the Origin of the epidemic".Nature.391(6667): 594–7.Bibcode:1998Natur.391..594Z.doi:10.1038/35400.PMID9468138.S2CID4416837.
- ^Kolata G (October 28, 1987)."Boy's 1969 death suggests AIDS invaded U.S. several times".The New York Times.RetrievedFebruary 11,2009.
- ^Chitnis A, Rawls D, Moore J (January 2000). "Origin of HIV type 1 in colonial French Equatorial Africa?".AIDS Research and Human Retroviruses.16(1): 5–8.doi:10.1089/088922200309548.PMID10628811.S2CID17783758.
- ^McNeil D Jr(September 16, 2010)."Precursor to H.I.V. was in monkeys for millennia".The New York Times.Archivedfrom the original on January 3, 2022.RetrievedSeptember 17,2010.
Dr. Marx believes that the crucial event was the introduction into Africa of millions of inexpensive, mass-produced syringes in the 1950s.... suspect that the growth of colonial cities is to blame. Before 1910, no Central African town had more than 10,000 people. But urban migration rose, increasing sexual contacts and leading to red-light districts.
- ^abWertheim JO, Worobey M (May 2009)."Dating the age of the SIV lineages that gave rise to HIV-1 and HIV-2".PLOS Computational Biology.5(5): e1000377.Bibcode:2009PLSCB...5E0377W.doi:10.1371/journal.pcbi.1000377.PMC2669881.PMID19412344.
Further reading
- Berlier W, Bourlet T, Lawrence P, Hamzeh H, Lambert C, Genin C, Verrier B, Dieu-Nosjean MC, Pozzetto B, Delézay O (2005). "Selective sequestration of X4 isolates by human genital epithelial cells: Implication for virus tropism selection process during sexual transmission of HIV".Journal of Medical Virology.77(4): 465–74.doi:10.1002/jmv.20478.PMID16254974.S2CID25762969.
- Joint United Nations Programme on HIV/AIDS(UNAIDS) (2011).Global HIV/AIDS Response, Epidemic update and health sector progress towards universal access(PDF).Joint United Nations Programme on HIV/AIDS.
- Muciaccia B, Padula F, Vicini E, Gandini L, Lenzi A, Stefanini M (2005)."Beta-chemokine receptors 5 and 3 are expressed on the head region of human spermatozoon".The FASEB Journal.19(14): 2048–50.doi:10.1096/fj.05-3962fje.hdl:11573/361629.PMID16174786.S2CID7928126.



