Hematite
| Hematite | |
|---|---|
 Braziliantrigonalhematite crystal | |
| General | |
| Category | Oxide minerals |
| Formula (repeating unit) | iron(III) oxide,Fe2O3,α-Fe2O3[1] |
| IMA symbol | Hem[2] |
| Strunz classification | 4.CB.05 |
| Dana classification | 4.3.1.2 |
| Crystal system | Trigonal |
| Crystal class | Hexagonal scalenohedral (3m) H–M symbol:(32/m) |
| Space group | R3c(no. 167) |
| Unit cell | a= 5.038(2) Å; c= 13.772(12) Å; Z = 6 |
| Identification | |
| Color | Metallic grey, dull to bright "rust-red" in earthy, compact, fine-grained material, steel-grey to black in crystals and massively crystalline ores |
| Crystal habit | Tabular to thick crystals; micaceous or platy, commonly in rosettes; radiating fibrous, reniform, botryoidal or stalactitic masses, columnar; earthy, granular, oolitic |
| Twinning | Penetration and lamellar |
| Cleavage | None, may show partings on {0001} and {1011} |
| Fracture | Uneven to subconchoidal |
| Tenacity | Brittle |
| Mohs scalehardness | 5.5–6.5 |
| Luster | Metallic to splendent |
| Streak | Bright red to dark red |
| Diaphaneity | Opaque |
| Specific gravity | 5.26 |
| Density | 5.3 |
| Optical properties | Uniaxial (−) |
| Refractive index | nω= 3.150–3.220,nε= 2.870–2.940 |
| Birefringence | δ= 0.280 |
| Pleochroism | O: brownish red; E: yellowish red |
| References | [3][4][5] |
Hematite(/ˈhiːməˌtaɪt,ˈhɛmə-/),also spelled ashaematite,is a commoniron oxidecompound with the formula,Fe2O3and is widely found inrocksandsoils.[6]Hematite crystals belong to therhombohedral lattice systemwhich is designated thealpha polymorphofFe
2O
3.It has the samecrystal structureascorundum(Al
2O
3) andilmenite(FeTiO
3). With this it forms a completesolid solutionat temperatures above 950 °C (1,740 °F).
Hematite naturally occurs in black to steel or silver-gray, brown to reddish-brown, or red colors. It isminedas an importantore mineral of iron.It is electrically conductive.[7]Hematite varieties includekidney ore,martite(pseudomorphsaftermagnetite),iron roseandspecularite(specularhematite). While these forms vary, they all have a rust-red streak. Hematite is not onlyharderthan pure iron, but also much morebrittle.Maghemiteis a polymorph of hematite (γ-Fe
2O
3) with the same chemical formula, but with aspinel structurelike magnetite.
Large deposits of hematite are found inbanded iron formations.Gray hematite is typically found in places that have still, standing water or mineralhot springs,such as those inYellowstone National ParkinNorth America.The mineral canprecipitatein the water and collect in layers at the bottom of the lake, spring, or other standing water. Hematite can also occur in the absence of water, usually as the result ofvolcanicactivity.
Clay-sized hematite crystals can also occur as a secondary mineral formed byweatheringprocesses insoil,and along with other iron oxides oroxyhydroxidessuch asgoethite,which is responsible for the red color of manytropical,ancient, or otherwise highly weathered soils.
Etymology and history[edit]
The name hematite is derived from theGreekword for blood,αἷμα(haima),due to the red coloration found in some varieties of hematite.[6]The color of hematite is often used as apigment.The English name of the stone is derived fromMiddle Frenchhématite pierre,which was taken fromLatinlapis haematitesc.the 15th century, which originated fromAncient Greekαἱματίτης λίθος(haimatitēs lithos,"blood-red stone" ).
Ochreis a clay that is colored by varying amounts of hematite, varying between 20% and 70%.[8]Red ochre contains unhydrated hematite, whereas yellow ochre containshydratedhematite (Fe2O3·H2O). The principal use of ochre is for tinting with a permanent color.[8]
Thered chalkwriting of this mineral was one of the earliest in the human history. The powdery mineral was first used 164,000 years ago by thePinnacle-Point man,possibly for social purposes.[9]Hematite residues are also found in graves from 80,000 years ago. NearRydnoinPolandandLovasinHungaryred chalk mines have been found that are from 5000 BC, belonging to theLinear Pottery cultureat theUpper Rhine.[10]
Rich deposits of hematite have been found on the island ofElbathat have been mined since the time of theEtruscans.[11]
Underground hematite mining is classified as carcinogenic hazard to humans.[12]
Magnetism[edit]
Hematite shows only a very feeble response to amagnetic field.Unlike magnetite, it is not noticeably attracted to an ordinary magnet. Hematite is anantiferromagneticmaterial below theMorin transitionat 250 K (−23 °C), and acantedantiferromagnet or weaklyferromagneticabove the Morin transition and below itsNéel temperatureat 948 K (675 °C), above which it isparamagnetic.
The magnetic structure of α-hematite was the subject of considerable discussion and debate during the 1950s, as it appeared to be ferromagnetic with a Curie temperature of approximately 1,000 K (730 °C), but with an extremely smallmagnetic moment(0.002Bohr magnetons). Adding to the surprise was a transition with a decrease in temperature at around 260 K (−13 °C) to a phase with no net magnetic moment. It was shown that the system is essentiallyantiferromagnetic,but that the low symmetry of thecationsites allowsspin–orbit couplingto causecanting of the momentswhen they are in the plane perpendicular to thecaxis. The disappearance of the moment with a decrease in temperature at 260 K (−13 °C) is caused by a change in theanisotropywhich causes the moments to align along thecaxis. In this configuration, spin canting does not reduce the energy.[13][14]The magnetic properties of bulk hematite differ from their nanoscale counterparts. For example, the Morin transition temperature of hematite decreases with a decrease in the particle size. The suppression of this transition has been observed in hematitenanoparticlesand is attributed to the presence of impurities, water molecules and defects in the crystals lattice. Hematite is part of a complex solid solution oxyhydroxide system having various contents of H2O (water), hydroxyl groups and vacancy substitutions that affect the mineral's magnetic and crystal chemical properties.[15]Two other end-members are referred to as protohematite and hydrohematite.
Enhancedmagnetic coercivitiesfor hematite have been achieved by dry-heating a two-line ferrihydrite precursor prepared from solution. Hematite exhibited temperature-dependent magnetic coercivity values ranging from 289 to 5,027oersteds(23–400 kA/m). The origin of these high coercivity values has been interpreted as a consequence of the subparticle structure induced by the different particle andcrystallitesize growth rates at increasing annealing temperature. These differences in the growth rates are translated into a progressive development of a subparticle structure at the nanoscale (super small). At lower temperatures (350–600 °C), single particles crystallize. However, at higher temperatures (600–1000 °C), the growth of crystalline aggregates, and a subparticle structure is favored.[16]
-
A microscopic picture of hematite
-
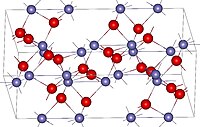
Crystal structure of hematite
Mine tailings[edit]
Hematite is present in the wastetailingsofiron mines.A recently developed process,magnetation,uses magnets to glean waste hematite from old mine tailings inMinnesota's vastMesabi Rangeiron district.[17]Falu redis a pigment used in traditional Swedish house paints. Originally, it was made from tailings of the Falu mine.[18]
Mars[edit]

The spectral signature of hematite was seen on the planetMarsby the infraredspectrometeron theNASAMars Global Surveyor[19]and2001 Mars Odyssey[20]spacecraft in orbit around Mars. The mineral was seen in abundance at two sites[21]on the planet, theTerra Meridianisite, near the Martian equator at 0° longitude, and theAram Chaossite near theValles Marineris.[22]Several other sites also showed hematite, such asAureum Chaos.[23]Because terrestrial hematite is typically a mineral formed in aqueous environments or by aqueous alteration, this detection was scientifically interesting enough that the second of the twoMars Exploration Roverswas sent to a site in the Terra Meridiani region designatedMeridiani Planum.In-situ investigations by theOpportunityrovershowed a significant amount of hematite, much of it in the form of small "Martian spherules"that were informally named" blueberries "by the science team. Analysis indicates that thesespherulesare apparentlyconcretionsformed from a water solution. "Knowing just how the hematite on Mars was formed will help us characterize the past environment and determine whether that environment was favorable for life".[24]
Jewelry[edit]
Hematite is often shaped into beads, tumbling stones, and other jewellery components.[25]Hematite was once used as mourning jewelry.[26][7]Certain types of hematite- or iron-oxide-rich clay, especiallyArmenian bole,have been used ingilding.Hematite is also used in art such as in the creation ofintaglio engraved gems.Hematineis a synthetic material sold asmagnetic hematite.[27]
Pigment[edit]
Hematite has been sourced to make pigments since earlier origins of human pictorial depictions, such as on cave linings and other surfaces, and has been continually employed in artwork through the eras. It forms the basis for red, purple and brown iron-oxide pigments, as well as being an important component of ochre, sienna and umber pigments.[28]
Industrial purposes[edit]
As mentioned earlier, hematite is an important mineral for iron ore. The physical properties of hematite are also employed in the areas of medical equipment, shipping industries and coal production. Having high density and capable as an effective barrier for X-ray passage, it is often incorporated into radiation shielding. As with other iron ores, it is often a component of ship ballasts for its density and economy. In the coal industry, it can be formed into a high specific density solution, to help separate coal powder from impurities.[29]
Gallery[edit]
-
A rare pseudo-scalenohedral crystal habit
-
Three gemmy quartz crystals containing bright rust-red inclusions of hematite, on a field of sparkly black specular hematite
-
Golden acicular crystals ofrutileradiating from a center of platy hematite
-
Cypro-Minoancylinder seal(left) made from hematite with corresponding impression (right), approximately 14th century BC
-
A cluster of parallel-growth, mirror-bright, metallic-gray hematite blades from Brazil
-
Hematite carving, 5 cm (2 in) long
-
Hematite, variant specularite (specular hematite), with fine grain shown
-
Red hematite frombanded iron formationinWyoming
-
Hematite on Mars as found in form of "blueberries" (named by NASA)
-
Streak plate,showing that Hematite consistently leaves a rust-red streak.
-
Hematite in Scanning Electron Microscope, magnification 100x.
-
Micaceous hematite taken with permission from Kelly's Mine, Lustleigh, Devon UK
See also[edit]
References[edit]
- ^Dunlop, David J.; Özdemir, Özden (2001).Rock Magnetism: Fundamentals and Frontiers.Cambridge: Cambridge University Press. p. 73.ISBN9780521000987.
- ^Warr, L.N. (2021)."IMA–CNMNC approved mineral symbols".Mineralogical Magazine.85(3): 291–320.Bibcode:2021MinM...85..291W.doi:10.1180/mgm.2021.43.S2CID235729616.
- ^Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C. (eds.)."Hematite"(PDF).Handbook of Mineralogy.Vol. III. Chantilly, VA: Mineralogical Society of America.ISBN978-0962209727.RetrievedDecember 22,2018.
- ^"Hematite Mineral Data".WebMineral.com.RetrievedDecember 22,2018.
- ^"Hematite".Mindat.org.RetrievedDecember 22,2018.
- ^abCornell, Rochelle M.; Schwertmann, Udo (1996).The Iron Oxides.Germany: Wiley. pp. 4, 26.ISBN9783527285761.LCCN96031931.RetrievedDecember 22,2018.
- ^abMorgenthau, Mengo L. (1923).Minerals and Cut Stones: Reference Book Containing Condensed and Simplified Descriptions from Standard Works on Mineralogy.p. 23.
- ^ab"Ochre".Industrial Minerals.Minerals Zone. Archived fromthe originalon November 15, 2016.RetrievedDecember 22,2018.
- ^"Researchers find earliest evidence for modern human behavior in South Africa"(Press release). AAAS. ASU News. October 17, 2007.RetrievedDecember 22,2018.
- ^Levato, Chiara (2016)."Iron Oxides Prehistoric Mines: A European Overview"(PDF).Anthropologica et Præhistorica.126:9–23.RetrievedDecember 22,2018.
- ^Benvenuti, M.; Dini, A.; D'Orazio, M.; Chiarantini, L.; Corretti, A.; Costagliola, P. (June 2013). "The tungsten and tin signature of iron ores from Elba Island (Italy)".Archaeometry.55(3): 479–506.doi:10.1111/j.1475-4754.2012.00692.x.
- ^https://monographs.iarc.who.int/list-of-classifications
- ^Dzyaloshinsky, I. E. (1958). "A thermodynamic theory of" weak "ferromagnetism of antiferromagnetics".Journal of Physics and Chemistry of Solids.4(4): 241–255.Bibcode:1958JPCS....4..241D.doi:10.1016/0022-3697(58)90076-3.
- ^Moriya, Tōru (1960)."Anisotropic Superexchange Interaction and Weak Ferromagnetism"(PDF).Physical Review.120(1): 91.Bibcode:1960PhRv..120...91M.doi:10.1103/PhysRev.120.91.
- ^Dang, M.-Z.; Rancourt, D. G.; Dutrizac, J. E.; Lamarche, G.; Provencher, R. (1998). "Interplay of surface conditions, particle size, stoichiometry, cell parameters, and magnetism in synthetic hematite-like materials".Hyperfine Interactions.117(1–4): 271–319.Bibcode:1998HyInt.117..271D.doi:10.1023/A:1012655729417.S2CID94031594.
- ^Vallina, B.; Rodriguez-Blanco, J. D.; Brown, A. P.; Benning, L. G.; Blanco, J. A. (2014)."Enhanced magnetic coercivity of α-Fe2O3obtained from carbonated 2-line ferrihydrite "(PDF).Journal of Nanoparticle Research.16(3): 2322.Bibcode:2014JNR....16.2322V.doi:10.1007/s11051-014-2322-5.S2CID137598876.
- ^Redman, Chris (May 20, 2009)."The next iron rush".Money.cnn.com.RetrievedDecember 22,2018.
- ^"Sveriges mest beprövade husfärg"[Sweden's most proven house color] (in Swedish).RetrievedDecember 22,2018.
- ^"Mars Global Surveyor TES Instrument Identification of Hematite on Mars"(Press release). NASA. May 27, 1998. Archived fromthe originalon May 13, 2007.RetrievedDecember 22,2018.
- ^Christensen, Philip R. (2004)."Formation of the hematite-bearing unit in Meridiani Planum: Evidence for deposition in standing water".Journal of Geophysical Research.109(E8): E08003.Bibcode:2004JGRE..109.8003C.doi:10.1029/2003JE002233.
- ^Bandfield, Joshua L. (2002)."Global mineral distributions on Mars"(PDF).Journal of Geophysical Research.107(E6): E65042.Bibcode:2002JGRE..107.5042B.doi:10.1029/2001JE001510.
- ^Glotch, Timothy D.; Christensen, Philip R. (2005)."Geologic and mineralogic mapping of Aram Chaos: Evidence for a water-rich history".Journal of Geophysical Research.110(E9): E09006.Bibcode:2005JGRE..110.9006G.doi:10.1029/2004JE002389.S2CID53489327.
- ^Glotch, Timothy D.; Rogers, D.; Christensen, Philip R. (2005)."A Newly Discovered Hematite-Rich Unit in Aureum Chaos: Comparison of Hematite and Associated Units With Those in Aram Chaos"(PDF).Lunar and Planetary Science.36:2159.Bibcode:2005LPI....36.2159G.
- ^"Hematite".NASA.RetrievedDecember 22,2018.
- ^"Hematite: A primary ore of iron and a pigment mineral".geology.com.Retrieved2023-09-07.
- ^Oldershaw, Cally (2003).Firefly Guide to Gems.Firefly Books. p. 53.ISBN978-1-55297-814-6.
- ^"Magnetic Hematite".Mindat.org.RetrievedDecember 22,2018.
- ^"Colors from the Earth: Violet Hematite".www.naturalpigments.com.Retrieved2023-09-07.
- ^"Hematite: A primary ore of iron and a pigment mineral".geology.com.Retrieved2023-09-07.