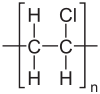
Polyvinyl chloride
| |

| |
 Pure PVC powder, containing no plasticizer
| |
| Names | |
|---|---|
| IUPAC name
poly(1-chloroethylene)[1]
| |
| Other names
Polychloroethene
| |
| Identifiers | |
| Abbreviations | PVC |
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.120.191 |
| KEGG | |
| MeSH | Polyvinyl+Chloride |
CompTox Dashboard(EPA)
|
|
| Properties | |
| (C2H3Cl)n[2] | |
| Appearance | white, brittle solid |
| Odor | odorless |
| Density | 1.4 g/cm3 |
| insoluble | |
| Solubilityinethanol | insoluble |
| Solubilityintetrahydrofuran | slightly soluble |
| −10.71×10−6(SI, 22 °C)[3] | |
| Hazards | |
| NFPA 704(fire diamond) | |
| 10 mg/m3(inhalable), 3 mg/m3(respirable) (TWA) | |
| NIOSH(US health exposure limits):[4] | |
PEL(Permissible)
|
15 mg/m3(inhalable), 5 mg/m3(respirable) (TWA) |
Except where otherwise noted, data are given for materials in theirstandard state(at 25 °C [77 °F], 100 kPa).
| |
| Elongation at break | 20–40% |
|---|---|
| Notch test | 2–5kJ/m2 |
| Glass Transition Temperature | 82 °C (180 °F)[5] |
| Melting point | 100 °C (212 °F) to 260 °C (500 °F)[5] |
| Effective heat of combustion | 17.95 MJ/kg |
| Specific heat(c) | 0.9kJ/(kg·K) |
| Water absorption (ASTM) | 0.04–0.4 |
| Dielectric Breakdown Voltage | 40 MV/m |
Polyvinyl chloride(alternatively:poly(vinyl chloride),[6][7]colloquial:vinyl[8]orpolyvinyl;abbreviated:PVC[8]) is the world's third-most widely produced syntheticpolymerofplastic(afterpolyethyleneandpolypropylene). About 40 million tons of PVC are produced each year.[9]
PVC comes in rigid (sometimes abbreviated as RPVC) and flexible forms. Rigid PVC is used inconstructionfor pipes, doors and windows. It is also used in making plastic bottles, packaging, and bank or membership cards. Addingplasticizersmakes PVC softer and more flexible. It is used in plumbing, electrical cable insulation, flooring, signage,phonograph records,inflatable products, and in rubber substitutes.[10]With cotton or linen, it is used in the production ofcanvas.
Polyvinyl chloride is a white, brittle solid. It is soluble inketones,chlorinated solvents,Dimethylformamide,THFandDMAc[11] .
Discovery
[edit]PVC was synthesized in 1872 by German chemistEugen Baumannafter extended investigation and experimentation.[12]The polymer appeared as a white solid inside a flask ofvinyl chloridethat had been left on a shelf sheltered from sunlight for four weeks. In the early 20th century, the Russian chemistIvan OstromislenskyandFritz Klatteof the German chemical company Griesheim-Elektron both attempted to use PVC in commercial products, but difficulties in processing the rigid, sometimes brittle polymer thwarted their efforts.Waldo Semonand theB.F. Goodrich Companydeveloped a method in 1926 toplasticizePVC by blending it with various additives,[13]including the use ofdibutyl phthalateby 1933.[14]
Production
[edit]Polyvinyl chloride is produced bypolymerizationof thevinyl chloridemonomer(VCM), as shown.[15]

|
About 80% of production involvessuspension polymerization.Emulsion polymerizationaccounts for about 12%, andbulk polymerizationaccounts for 8%. Suspension polymerization produces particles with average diameters of 100–180 μm, whereas emulsion polymerization gives much smaller particles of average size around 0.2 μm. VCM and water are introduced into the reactor along with a polymerization initiator and other additives. The contents of the reaction vessel are pressurized and continually mixed to maintain the suspension and ensure a uniform particle size of the PVC resin. The reaction isexothermicand thus requires cooling. As the volume is reduced during the reaction (PVC is denser than VCM), water is continually added to the mixture to maintain the suspension.[9]
PVC may be manufactured fromethylene,which can be produced from eithernaphthaorethanefeedstock.[16]
Microstructure
[edit]Thepolymersare linear and are strong. Themonomersare mainly arranged head-to-tail, meaning thatchlorideis located on alternating carbon centres. PVC has mainly anatactic stereochemistry,which means that the relativestereochemistryof the chloride centres are random. Some degree ofsyndiotacticityof the chain gives a few percent crystallinity that is influential on the properties of the material. About 57% of the mass of PVC ischlorine.The presence of chloride groups gives the polymer very different properties from the structurally related materialpolyethylene.[17]At 1.4 g/cm3,PVC's density is also higher than structurally related plastics such aspolyethylene(0.88–0.96 g/cm3) andpolymethylmethacrylate(1.18 g/cm3).
Producers
[edit]About half of the world's PVC production capacity is inChina,despite the closure of many Chinese PVC plants due to issues complying with environmental regulations and poor capacities of scale. The largest single producer of PVC as of 2018 isShin-Etsu ChemicalofJapan,with a global share of around 30%.[16]
Additives
[edit]The product of the polymerization process is unmodified PVC. Before PVC can be made into finished products, it always requires conversion into a compound by the incorporation of additives (but not necessarily all of the following) such asheat stabilizers,UV stabilizers,plasticizers, processing aids, impact modifiers, thermal modifiers, fillers,flame retardants,biocides,blowing agents and smoke suppressors, and, optionally, pigments.[18]The choice of additives used for the PVC finished product is controlled by the cost performance requirements of the end use specification (underground pipe, window frames, intravenous tubing and flooring all have very different ingredients to suit their performance requirements). Previously,polychlorinated biphenyls(PCBs) were added to certain PVC products as flame retardants and stabilizers.[19]
Plasticizers
[edit]Among thecommon plastics,PVC is unique in its acceptance of large amounts of plasticizer with gradual changes in physical properties from a rigid solid to a soft gel,[20]and almost 90% of all plasticizer production is used in making flexible PVC.[21][22]The majority is used in films and cable sheathing.[23]Flexible PVC can consist of over 85% plasticizer by mass, however unplasticized PVC (UPVC) should not contain any.[24]
| Plasticizer content (%DINPby weight) | Specific gravity(20 °C) | Shore hardness (type A, 15 s) |
Flexural stiffness(Mpa) | Tensile strength(Mpa) | Elongation at break (%) | Example applications | |
|---|---|---|---|---|---|---|---|
| Rigid | 0 | 1.4 | 900 | 41 | <15 | Unplasticized PVC (UPVC): window frames and sills, doors,rigid pipe | |
| Semi-rigid | 25 | 1.26 | 94 | 69 | 31 | 225 | Vinyl flooring,flexible pipe, thin films (stretch wrap), advertising banners |
| Flexible | 33 | 1.22 | 84 | 12 | 21 | 295 | Wire and cable insulation, flexible pipe |
| Very Flexible | 44 | 1.17 | 66 | 3.4 | 14 | 400 | Boots and clothing, inflatables, |
| Extremely Flexible | 86 | 1.02 | < 10 | Fishing lures (soft plastic bait),polymer clay,plastisolinks |
Phthalates
[edit]The most common class of plasticizers used in PVC is phthalates, which are diesters ofphthalic acid.Phthalates can be categorized as high and low, depending on their molecular weight. Low phthalates such asBis(2-ethylhexyl) phthalate(DEHP) andDibutyl phthalate(DBP) have increased health risks and are generally being phased out. High-molecular-weight phthalates such asdiisononyl phthalate(DINP) anddiisodecyl phthalate(DIDP) are generally considered safer.[22]
While DEHP has been medically approved for many years for use in medical devices, it was permanently banned for use in children's products in the US in 2008 by US Congress;[25]the PVC-DEHP combination had proved to be very suitable for making blood bags because DEHP stabilizes red blood cells, minimizinghemolysis(red blood cell rupture). However, DEHP is coming under increasing pressure in Europe. The assessment of potential risks related to phthalates, and in particular the use of DEHP in PVC medical devices, was subject to scientific and policy review by the European Union authorities, and on 21 March 2010, a specific labeling requirement was introduced across the EU for all devices containing phthalates that are classified as CMR (carcinogenic, mutagenic or toxic to reproduction).[26]The label aims to enable healthcare professionals to use this equipment safely, and, where needed, take appropriate precautionary measures for patients at risk of over-exposure[27]

Metal stabilizers
[edit]BaZn stabilisers have successfully replaced cadmium-based stabilisers in Europe in many PVC semi-rigid and flexible applications.[28]
In Europe, particularly Belgium, there has been a commitment to eliminate the use of cadmium (previously used as a part component of heat stabilizers in window profiles) and phase out lead-based heat stabilizers (as used in pipe and profile areas) such as liquid autodiachromate and calcium polyhydrocummate by 2015. According to the final report ofVinyl 2010,[29]cadmium was eliminated across Europe by 2007. The progressive substitution of lead-based stabilizers is also confirmed in the same document showing a reduction of 75% since 2000 and ongoing. This is confirmed by the corresponding growth in calcium-based stabilizers, used as an alternative to lead-based stabilizers, more and more, also outside Europe.[9]
Heat stabilizers
[edit]Some of the most crucial additives are heat stabilizers. These agents minimizeloss of HCl,a degradation process that starts above 70 °C (158 °F) and isautocatalytic.Many diverse agents have been used including, traditionally, derivatives ofheavy metals(lead, cadmium). Metallic soaps (metal "salts" offatty acidssuch ascalcium stearate) are common in flexible PVC applications.[9]
Properties
[edit]PVC is athermoplasticpolymer. Its properties are usually categorized based on rigid and flexible PVCs.[30]
| Property | Unit of measurement | Rigid PVC | Flexible PVC |
|---|---|---|---|
| Density[31] | g/cm3 | 1.3–1.45 | 1.1–1.35 |
| Thermal conductivity[32] | W/(m·K) | 0.14–0.28 | 0.14–0.17 |
| Yield strength[31] | psi | 4,500–8,700 | 1,450–3,600 |
| MPa | 31–60 | 10.0–24.8 | |
| Young's modulus[33] | psi | 490,000 | — |
| GPa | 3.4 | — | |
| Flexural strength(yield)[33] | psi | 10,500 | — |
| MPa | 72 | — | |
| Compression strength[33] | psi | 9,500 | — |
| MPa | 66 | — | |
| Coefficient of thermal expansion(linear)[33] | mm/(mm °C) | 5×10−5 | — |
| Vicat B[32] | °C | 65–100 | Not recommended |
| Resistivity[a][34] | Ωm | 1016 | 1012–1015 |
| Surface resistivity[a][34] | Ω | 1013–1014 | 1011–1012 |
- Notes
Thermal and fire
[edit]Theheat stabilityof raw PVC is very poor, so the addition of a heat stabilizer during the process is necessary in order to ensure the product's properties. Traditional product PVC has a maximum operating temperature around 60 °C (140 °F) when heat distortion begins to occur.[35]
As a thermoplastic, PVC has an inherent insulation that aids in reducing condensation formation and resisting internal temperature changes for hot and cold liquids.[35]
Applications
[edit]
Pipes
[edit]Roughly half of the world's PVC resin manufactured annually is used for producingpipesfor municipal and industrial applications.[36]In the private homeowner market, it accounts for 66% of the household market in the US, and in household sanitary sewer pipe applications, it accounts for 75%.[37][38]Buried PVC pipes in both water and sanitary sewer applications that are 100 mm (4 in) in diameter and larger are typically joined by means of a gasket-sealed joint. The most common type of gasket utilized in North America is a metal-reinforced elastomer, commonly referred to as a Rieber sealing system.[39]
Electric cables
[edit]PVC is often used as theinsulatingsheath onelectrical cables.PVC is chosen because of its good electrical insulation, ease ofextrusion,and resistance to burn.[40]
In a fire, PVC can formhydrogen chloridefumes; the chlorine serves to scavengefree radicals,making PVC-coated wiresfire retardant.While hydrogen chloride fumes can also pose ahealth hazardin their own right, it dissolves in moisture and breaks down onto surfaces, particularly in areas where the air is cool enough to breathe, so would not be inhaled.[41]
Construction
[edit]
PVC is widely and heavily used in construction and building industry,[9]For example,vinyl sidingis extensively is a popular low-maintenance material, particularly inIreland,the United Kingdom, the United States, and Canada. The material comes in a range of colors and finishes, including a photo-effect wood finish, and is used as a substitute for painted wood, mostly for window frames andsillswhen installinginsulated glazingin new buildings; or to replace older single-glazed windows, as it does not decompose and is weather-resistant. Other uses includefascia,andsidingorweatherboarding.This material has almost entirely replaced the use ofcast ironforplumbinganddrainage,being used for waste pipes, drainpipes,guttersanddownspouts.PVC is known as having strong resistance against chemicals, sunlight, and oxidation from water.[42]

Signage and graphics
[edit]Polyvinyl chloride is formed in flat sheets in a variety of thicknesses and colors. As flat sheets, PVC is often expanded to create voids in the interior of the material, providing additional thickness without additional weight and minimal extra cost (seeclosed-cell PVC foamboard). Sheets are cut using saws and rotary cutting equipment.
Plasticized PVC is also used to produce thin, colored, or clear,adhesive-backed films referred to simply as "vinyl". These films are typically cut on acomputer-controlledplotter(seevinyl cutter) or printed in awide-format printer.These sheets and films are used to produce a wide variety ofcommercial signageproducts,vinyl wrapsorracing stripeson vehicles for aesthetics or aswrap advertising,and general purposestickers.[43]
Clothing
[edit]
PVC fabriciswater-resistant,used for its weather-resistant qualities in coats, skiing equipment, shoes,jackets,andaprons.[citation needed]
Healthcare
[edit]The two main application areas forsingle-usemedically approved PVC compounds are flexible containers and tubing: containers used for blood and blood components, for urine collection or for ostomy products and tubing used for blood taking and blood giving sets, catheters, heart-lung bypass sets, hemodialysis sets etc. In Europe the consumption of PVC from medical devices is approximately 85,000 tons each year. Almost one third of plastic-based medical devices are made from PVC.[44]
Food packaging
[edit]PVC has been applied to various items such as: bottles,[45]packagingfilms,[45]blister packs,[45]cling wraps,[45]and seals on metal lids.
Wire rope
[edit]PVC may beextrudedunder pressure to encasewire ropeand aircraft cable used for general purpose applications. PVC coated wire rope is easier to handle, resists corrosion and abrasion, and may be color-coded for increased visibility. It is found in a variety of industries and environments both indoor and out.[46]
Other uses
[edit]
Molded PVC is used to producephonograph, or "vinyl," records.PVC piping is a cheaper alternative to metal tubing used in musical instrument making; it is therefore a common alternative when making wind instruments, often for leisure or for rarer instruments such as thecontrabass flute.An instrument that is almost exclusively built from PVC tube is thethongophone,a percussion instrument that is played by slapping the open tubes with aflip-flopor similar.[47]PVC is also used as a raw material in automotive underbody coating.[48]
Chlorinated PVC
[edit]PVC can be usefully modified by chlorination, which increases its chlorine content to or above 67%.Chlorinated polyvinyl chloride,(CPVC), as it is called, is produced by chlorination of aqueous solution of suspension PVC particles followed by exposure toUV lightwhich initiates the free-radical chlorination.[9]
Health and safety
[edit]Plasticizers
[edit]Phthalates, which are incorporated into plastics as plasticizers, comprise approximately 70% of the US plasticizer market; phthalates are by design not covalently bound to the polymer matrix, which makes them highly susceptible to leaching. Phthalates are contained in plastics at high percentages. For example, they can contribute up to 40% by weight to intravenous medical bags and up to 80% by weight in medical tubing.[49]Vinyl products are pervasive—including toys,[50]car interiors, shower curtains, and flooring—and initially release chemical gases into the air. Some studies indicate that thisoutgassingof additives may contribute to health complications, and have resulted in a call for banning the use of DEHP on shower curtains, among other uses.[51]
In 2004 a joint Swedish-Danish research team found a statistical association between allergies in children and indoor air levels of DEHP and BBzP (butyl benzyl phthalate), which is used in vinyl flooring.[52]In December 2006, theEuropean Chemicals Bureauof the European Commission released a final draft risk assessment of BBzP which found "no concern" for consumer exposure including exposure to children.[53]
Lead
[edit]Leadcompounds had previously been widely added to PVC to improve workability and stability but have been shown to leach into drinking water from PVC pipes.[54]
In Europe the use of lead-based stabilizers has been discontinued. TheVinylPlusvoluntary commitment which began in 2000, saw European Stabiliser Producers Association (ESPA) members complete the replacement of Pb-based stabilisers in 2015.[55][56]
Vinyl chloride monomer
[edit]In the early 1970s, the carcinogenicity of vinyl chloride (usually called vinyl chloride monomer or VCM) was linked to cancers in workers in the polyvinyl chloride industry. Specifically workers in polymerization section of aB.F. Goodrichplant nearLouisville, Kentucky,were diagnosed with liverangiosarcomaalso known ashemangiosarcoma,a rare disease.[57]Since that time, studies of PVC workers in Australia, Italy, Germany, and the UK have all associated certain types of occupational cancers with exposure to vinyl chloride, and it has become accepted that VCM is a carcinogen.[9]
Combustion
[edit]PVC producesHCland carbon dioxide upon combustion.
Dioxins
[edit]Studies of household waste burning indicate consistent increases in dioxin generation with increasing PVC concentrations.[58]According to the U.S. EPA dioxin inventory,landfill firesare likely to represent an even larger source of dioxin to the environment. A survey of international studies consistently identifies high dioxin concentrations in areas affected by open waste burning and a study that looked at the homologue pattern found the sample with the highest dioxin concentration was "typical for the pyrolysis of PVC". Other EU studies indicate that PVC likely "accounts for the overwhelming majority of chlorine that is available for dioxin formation during landfill fires."[58]
The next largest sources of dioxin in the U.S. EPA inventory are medical and municipal waste incinerators.[59]Various studies have been conducted that reach contradictory results. For instance a study of commercial-scale incinerators showed no relationship between the PVC content of the waste and dioxin emissions.[60][61]Other studies have shown a clear correlation between dioxin formation and chloride content and indicate that PVC is a significant contributor to the formation of both dioxin and PCB in incinerators.[62][63][64]
In February 2007, the Technical and Scientific Advisory Committee of theUS Green Building Council(USGBC) released its report on a PVC avoidance related materials credit for theLEEDGreen Building Rating system. The report concludes that "no single material shows up as the best across all the human health and environmental impact categories, nor as the worst" but that the "risk of dioxin emissions puts PVC consistently among the worst materials for human health impacts."[65]
In Europe the overwhelming importance of combustion conditions on dioxin formation has been established by numerous researchers. The single most important factor in forming dioxin-like compounds is the temperature of the combustion gases. Oxygen concentration also plays a major role on dioxin formation, but not the chlorine content.[66]
Several studies have also shown that removing PVC from waste would not significantly reduce the quantity of dioxins emitted. The EU Commission published in July 2000 a Green Paper on the Environmental Issues of PVC "[67]
A study commissioned by the European Commission on "Life Cycle Assessment of PVC and of principal competing materials" states that "Recent studies show that the presence of PVC has no significant effect on the amount of dioxins released through incineration ofplastic waste."[68]
Industry initiatives
[edit]In Europe, developments in PVC waste management have been monitored by Vinyl 2010,[69]established in 2000. Vinyl 2010's objective was to recycle 200,000 tonnes of post-consumer PVC waste per year in Europe by the end of 2010, excluding waste streams already subject to other or more specific legislation (such as the European Directives onEnd-of-Life Vehicles,Packaging and Waste Electric and Electronic Equipment).[citation needed]
Since June 2011, it is followed by VinylPlus, a new set of targets for sustainable development.[70]Its main target is to recycle 800,000 tonnes per year of PVC by 2020 including 100,000 tonnes of "difficult to recycle" waste. One facilitator for collection and recycling of PVC waste is Recovinyl.[71]The reported and audited mechanically recycled PVC tonnage in 2016 was 568,695 tonnes which in 2018 had increased to 739,525 tonnes.[72]
One approach to address the problem of waste PVC is also through the process calledVinyloop.It is a mechanical recycling process using a solvent to separate PVC from other materials. This solvent turns in a closed loop process in which the solvent is recycled. Recycled PVC is used in place of virgin PVC in various applications: coatings for swimming pools, shoe soles, hoses, diaphragms tunnel, coated fabrics, PVC sheets.[73]This recycled PVC's primary energy demand is 46 percent lower than conventional produced PVC. So the use of recycled material leads to a significant betterecological footprint.Theglobal warming potentialis 39 percent lower.[74]
Restrictions
[edit]In November 2005, one of the largest hospital networks in the US,Catholic Healthcare West,signed a contract withB. BraunMelsungen for vinyl-free intravenous bags and tubing.[75]
In January 2012, a major US West Coast healthcare provider,Kaiser Permanente,announced that it will no longer buy intravenous (IV) medical equipment made with PVC and DEHP-type plasticizers.[76]
In 1998, theU.S. Consumer Product Safety Commission(CPSC) arrived at a voluntary agreement with manufacturers to remove phthalates from PVC rattles, teethers, baby bottle nipples and pacifiers.[77]
Vinyl gloves in medicine
[edit]
Plasticized PVC is a common material formedical gloves.Due to vinyl gloves having less flexibility and elasticity, several guidelines recommend eitherlatexornitrilegloves for clinical care and procedures that require manual dexterity and/or that involve patient contact for more than a brief period. Vinyl gloves show poor resistance to many chemicals, including glutaraldehyde-based products and alcohols used in formulation of disinfectants for swabbing down work surfaces or in hand rubs. The additives in PVC are also known to cause skin reactions such as allergic contact dermatitis. These are for example the antioxidantbisphenol A,the biocidebenzisothiazolinone,propylene glycol/adipate polyester and ethylhexylmaleate.[78]
Sustainability
[edit]The life cycle, sustainability, and appropriateness of PVC are discussed.[79][by whom?]In Europe, a 2021 VinylPlus Progress Report indicated that 731,461 tonnes PVC were recycled in 2020, a 5% reduction compared to 2019 due to theCOVID-19 pandemic.[80]
See also
[edit]References
[edit]General references
[edit]- Titow, W. (1984).PVC Technology.London: Elsevier Applied Science Publishers.ISBN978-0-85334-249-6.
Inline citations
[edit]- ^"poly(vinyl chloride) (CHEBI:53243)".CHEBI.Archivedfrom the original on 13 December 2013.Retrieved12 July2012.
- ^"Substance Details CAS Registry Number: 9002-86-2".Commonchemistry.CAS.Archivedfrom the original on 21 May 2018.Retrieved12 July2012.
- ^Wapler, M. C.; Leupold, J.; Dragonu, I.; von Elverfeldt, D.; Zaitsev, M.; Wallrabe, U. (2014). "Magnetic properties of materials for MR engineering, micro-MR and beyond".JMR.242:233–242.arXiv:1403.4760.Bibcode:2014JMagR.242..233W.doi:10.1016/j.jmr.2014.02.005.PMID24705364.S2CID11545416.
- ^"Material Safety Data Sheet: PVC Compounds Pellet and Powder"(PDF).Georgia Gulf Chemical and Vinyls LLC.Archived(PDF)from the original on 17 August 2021.Retrieved23 July2021.
- ^abWilkes, Charles E.; Summers, James W.; Daniels, Charles Anthony; Berard, Mark T. (2005).PVC Handbook.Hanser Verlag. p. 414.ISBN978-1-56990-379-7.Archivedfrom the original on 17 November 2016.Retrieved24 September2016.
- ^"Poly(vinyl chloride)".MilliporeSigma. 2022.Archivedfrom the original on 11 October 2022.Retrieved11 October2022.
- ^"Poly(Vinyl Chloride)".
- ^ab"About PVC".The European Council of Vinyl Manufacturers.Archivedfrom the original on 5 December 2023.Retrieved17 March2024.
- ^abcdefgAllsopp, M. W.; Vianello, G. (2012). "Poly(Vinyl Chloride)".Ullmann's Encyclopedia of Industrial Chemistry.Weinheim: Wiley-VCH.doi:10.1002/14356007.a21_717.ISBN978-3527306732.
- ^W. V. Titow (31 December 1984).PVC technology.Springer. pp. 6–.ISBN978-0-85334-249-6.Archivedfrom the original on 26 May 2013.Retrieved6 October2011.
- ^Grause, Guido; Hirahashi, Suguru; Toyoda, Hiroshi; Kameda, Tomohito; Yoshioka, Toshiaki (2017). "Solubility parameters for determining optimal solvents for separating PVC from PVC-coated PET fibers".Journal of Material Cycles and Waste Management.19:612–622.doi:10.1007/s10163-015-0457-9.
- ^Baumann, E. (1872)"Ueber einige Vinylverbindungen"Archived17 November 2016 at theWayback Machine(On some vinyl compounds),Annalen der Chemie und Pharmacie,163:308–322.
- ^Semon, Waldo L.; Stahl, G. Allan (April 1981). "History of Vinyl Chloride Polymers".Journal of Macromolecular Science: Part A - Chemistry.15(6): 1263–1278.doi:10.1080/00222338108066464.
- ^US 1929453,Waldo Semon, "Synthetic rubber-like composition and method of making same", published 1933-10-10, assigned to B.F. GoodrichArchived26 April 2022 at theWayback Machine
- ^Chanda, Manas; Roy, Salil K. (2006).Plastics technology handbook.CRC Press. pp. 1–6.ISBN978-0-8493-7039-7.
- ^ab"Shin-Etsu Chemical to build $1.4bn polyvinyl chloride plant in US".Nikkei Asian Review.Archivedfrom the original on 24 July 2018.Retrieved24 July2018.
- ^Handbook of Plastics, Elastomers, and Composites, Fourth Edition, 2002 by The McGraw-Hill, Charles A. Harper Editor-in-Chief.ISBN0-07-138476-6
- ^David F. Cadogan and Christopher J. Howick "Plasticizers" in Ullmann's Encyclopedia of Industrial Chemistry 2000, Wiley-VCH, Weinheim.doi:10.1002/14356007.a20_439
- ^Karlen, Kaley."Health Concerns and Environmental Issues with PVC-Containing Building Materials in Green Buildings"(PDF).Integrated Waste Management Board.California Environmental Protection Agency, US.Archived(PDF)from the original on 5 February 2016.Retrieved26 August2015.
- ^Krauskopf, Leonard G. (2009). "3.13 Plasticizers".Plastics additives handbook(6. ed.). Munich: Carl Hanser Verlag. pp. 485–511.ISBN978-3-446-40801-2.
- ^David F. Cadogan and Christopher J. Howick "Plasticizers" in Ullmann's Encyclopedia of Industrial Chemistry 2000, Wiley-VCH, Weinheim.doi:10.1002/14356007.a20_439
- ^ab"factsheets - Plasticisers - Information Center".Plasticisers.Archivedfrom the original on 9 February 2022.Retrieved19 February2022.
- ^"Plasticizers Market Report".Ceresana.Retrieved7 January2023.
- ^abKrauskopf, L. G. (2009).Plastics additives handbook(6. ed.). Munich:Carl Hanser Verlag.p. 495.ISBN978-3-446-40801-2.
- ^"Phthalates and DEHP".Health Care Without Harm. 29 April 2013.Retrieved23 July2021.[permanent dead link]
- ^Opinion on The safety of medical devices containing DEHP plasticized PVC or other plasticizers on neonates and other groups possibly at risk (2015 update)Archived3 February 2016 at theWayback Machine.Scientific Committee on Emerging and Newly-Identified Health Risks (25 June 2015).
- ^"You searched for DEHP - Plasticisers - Information Center".Plasticisers.Archivedfrom the original on 9 February 2022.Retrieved19 February2022.
- ^Liquid stabilisers.Seuropean Stabiliser Producers Association
- ^Vinyl 2010.The European PVC Industry's Sustainable Development Programme
- ^"DIFFERENCES BETWEEN FLEXIBLE AND RIGID PVC COMPOUNDS".Green PVC. 12 August 2021.Archivedfrom the original on 16 December 2021.
- ^abTitow 1984,p. 1186.
- ^abTitow 1984,p. 1191.
- ^abcdTitow 1984,p. 857.
- ^abTitow 1984,p. 1194.
- ^abMichael A. Joyce, Michael D. Joyce (2004).Residential Construction Academy: Plumbing.Cengage Learning. pp. 63–64.
- ^Rahman, Shah (19–20 June 2007).PVC Pipe & Fittings: Underground Solutions for Water and Sewer Systems in North America(PDF).2nd Brazilian PVC Congress, Sao Paulo, Brazil. Archived fromthe original(PDF)on 9 July 2015.Retrieved28 February2009.
- ^Uses for vinyl: pipe.vinylbydesign.com
- ^Rahman, Shah (October 2004)."Thermoplastics at Work: A Comprehensive Review of Municipal PVC Piping Products"(PDF).Underground Construction:56–61.Archivedfrom the original on 7 August 2020.Retrieved5 February2019.
- ^Shah Rahman (April 2007)."Sealing Our Buried Lifelines"(PDF).Opflow.33(4): 12–17.Bibcode:2007Opflo..33d..12R.doi:10.1002/j.1551-8701.2007.tb02753.x.Archived(PDF)from the original on 8 October 2011.Retrieved30 March2010.
- ^Titow 1984,p. 717PVC coating of wire and cable
- ^Galloway FM, Hirschler MM, Smith GF (1992). "Surface parameters from small-scale experiments used for measuring HCl transport and decay in fire atmospheres".Fire Mater.15(4): 181–189.doi:10.1002/fam.810150405.
- ^Strong, A. Brent (2005)Plastics: Materials and Processing.Prentice Hall. pp. 36–37, 68–72.ISBN0131145584.
- ^Ellis, R."Vinyl: an Honest Conversation".Archivedfrom the original on 28 January 2021.Retrieved3 June2020.
- ^PVC Healthcare Applications.pvcmed.org
- ^abcdMarsh, Kenneth; Bigusu, Betty (31 March 2007)."Food Packaging – Roles, Materials, and Environmental Issues".Journal of Food Science.72(3): R43.doi:10.1111/j.1750-3841.2007.00301.x.ISSN1750-3841.PMID17995809.
- ^"Coated Aircraft Cable & Wire Rope".Lexco Cable.Archivedfrom the original on 26 August 2017.Retrieved25 August2017.
- ^Building a PVC Instrument.natetrue.com
- ^Takata, Ayumi; Ohashi, Yutaka (2002)."Post PVC Sound Insulating Underbody coating".SAE Technical Paper Series.Vol. 1.doi:10.4271/2002-01-0293.
- ^Halden, Rolf U. (2010)."Plastics and Health Risks".Annual Review of Public Health.31(1): 179–194.doi:10.1146/annurev.publhealth.012809.103714.PMID20070188.
- ^Directive 2005/84/EC of the European Parliament and of the Council 14 December 2005Archived4 May 2013 at theWayback Machine.Official Journal of the European Union. 27 December 2005
- ^Vinyl shower curtains a 'volatile' hazard, study saysArchived4 September 2010 at theWayback Machine.Canada.com (12 June 2008). Retrieved on 6 October 2011.
- ^Bornehag, Carl-Gustaf; Sundell, Jan; Weschler, Charles J.; Sigsgaard, Torben; Lundgren, Björn; Hasselgren, Mikael; Hägerhed-Engman, Linda; et al. (2004)."The Association between Asthma and Allergic Symptoms in Children and Phthalates in House Dust: A Nested Case–Control Study".Environmental Health Perspectives.112(14): 1393–1397.doi:10.1289/ehp.7187.PMC1247566.PMID15471731.
- ^Phthalate Information Center Blog: More good news from Europe.phthalates.org (3 January 2007)
- ^"China's PVC pipe makers under pressure to give up lead stabilizers".6 September 2013.
- ^"Lead replacement".European Stabiliser Producers Association.Archivedfrom the original on 5 December 2018.Retrieved5 December2018.
- ^"VinylPlus Progress Report 2016"(PDF).VinylPlus.30 April 2016.Archived(PDF)from the original on 20 December 2016.
- ^Creech, J. L. Jr.; Johnson, M. N. (March 1974). "Angiosarcoma of liver in the manufacture of polyvinyl chloride".Journal of Occupational Medicine.16(3): 150–1.PMID4856325.
- ^abCostner, Pat (2005)"Estimating Releases and Prioritizing Sources in the Context of the Stockholm Convention"Archived27 September 2007 at theWayback Machine,International POPs Elimination Network, Mexico.
- ^Beychok, M.R. (1987). "A data base of dioxin and furan emissions from municipal refuse incinerators".Atmospheric Environment.21(1): 29–36.Bibcode:1987AtmEn..21...29B.doi:10.1016/0004-6981(87)90267-8.
- ^National Renewable Energy Laboratory,Polyvinyl Chloride Plastics in Municipal Solid Waste CombustionArchived15 February 2013 at theWayback MachineNREL/TP-430- 5518, Golden CO, April 1993
- ^Rigo, H. G.; Chandler, A. J.; Lanier, W.S. (1995).The Relationship between Chlorine in Waste Streams and Dioxin Emissions from Waste Combustor Stacks(PDF).Vol. 36. New York, NY: American Society of Mechanical Engineers.ISBN978-0-7918-1222-8.Archived fromthe original(PDF)on 7 April 2016.Retrieved31 October2009.
{{cite book}}:|journal=ignored (help) - ^Katami, Takeo; Yasuhara, Akio; Okuda, Toshikazu; Shibamoto, Takayuki; et al. (2002). "Formation of PCDDs, PCDFs, and Coplanar PCBs from Polyvinyl Chloride during Combustion in an Incinerator".Environ. Sci. Technol.36(6): 1320–1324.Bibcode:2002EnST...36.1320K.doi:10.1021/es0109904.PMID11944687.
- ^Wagner, J.; Green, A. (1993). "Correlation of chlorinated organic compound emissions from incineration with chlorinated organic input".Chemosphere.26(11): 2039–2054.Bibcode:1993Chmsp..26.2039W.doi:10.1016/0045-6535(93)90030-9.
- ^Thornton, Joe (2002).Environmental Impacts of polyvinyl Chloride Building Materials(PDF).Washington, DC:Healthy Building Network.ISBN978-0-9724632-0-1.Archived fromthe original(PDF)on 20 September 2013.Retrieved6 October2011.
- ^The USGBC documentArchived13 July 2007 at theWayback Machine;An analysis by the Healthy Building NEtworkArchived2 June 2008 at theWayback Machine
- ^Wikstrom, Evalena; G. Lofvenius; C. Rappe; S. Marklund (1996). "Influence of Level and Form of Chlorine on the Formation of Chlorinated Dioxins, Dibenzofurans, and Benzenes during Combustion of an Artificial Fuel in a Laboratory Reactor".Environmental Science & Technology.30(5): 1637–1644.Bibcode:1996EnST...30.1637W.doi:10.1021/es9506364.
- ^Environmental issues of PVCArchived12 May 2012 at theWayback Machine.European Commission. Brussels, 26 July 2000
- ^Life Cycle Assessment of PVC and of principal competing materials Commissioned by the European Commission.European Commission (July 2004), p. 96
- ^Home – Vinyl 2010 The European PVC industry commitment to SustainabilityArchived25 July 2013 at theWayback Machine.Vinyl2010.org (22 June 2011). Retrieved on 6 October 2011.
- ^Our Voluntary Commitment.vinylplus.eu
- ^Incentives to collect and recycleArchived19 January 2022 at theWayback Machine.Recovinyl.com. Retrieved on 28 January 2016.
- ^"VinylPlus Progress Report 2019"(PDF).Archived(PDF)from the original on 14 February 2020.Retrieved22 September2019.
- ^Solvay, asking more from chemistryArchived1 January 2012 at theWayback Machine.Solvayplastics.com (15 July 2013). Retrieved on 28 January 2016.
- ^Solvay, asking more from chemistryArchived16 May 2016 at the Portuguese Web Archive. Solvayplastics.com (15 July 2013). Retrieved on 28 January 2016.
- ^"CHW Switches to PVC/DEHP-Free Products to Improve Patient Safety and Protect the Environment".Business Wire.21 November 2005.Archivedfrom the original on 9 April 2016.Retrieved28 January2016.
- ^Smock, Doug (19 January 2012)Kaiser Permanente bans PVC tubing and bags.plasticstoday.com
- ^"PVC Policies Across the World".chej.org.Archivedfrom the original on 10 August 2017.Retrieved25 August2017.
- ^"Vinyl Gloves: Causes For Concern"(PDF).Ansell(glove manufacturer). Archived fromthe original(PDF)on 22 September 2015.Retrieved17 November2015.
- ^London 2012 Use of PVC PolicyArchived1 February 2016 at theWayback Machine.independent.gov.uk.
- ^"VinylPlus at a Glance 2021 - VinylPlus".Vinylplus.eu. 17 May 2021.Archivedfrom the original on 7 February 2022.Retrieved19 February2022.



