Jablonski diagram

This articleneeds additional citations forverification.(July 2023) |
In molecularspectroscopy,aJablonski diagramis a diagram that illustrates theelectronic statesand often thevibrational levelsof amolecule,and also the transitions between them. The states are arranged vertically by energy and grouped horizontally byspin multiplicity.[1]Nonradiative transitionsare indicated by squiggly arrows andradiative transitionsby straight arrows. The vibrational ground states of each electronic state are indicated with thick lines, the higher vibrational states with thinner lines.[2] The diagram is named after the Polish physicistAleksander Jabłońskiwho first proposed it in 1933.[3]
Transitions[edit]
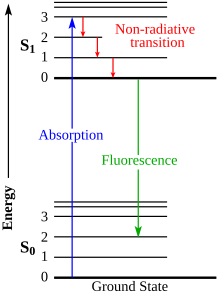
When a moleculeabsorbs a photon,the photon energy is converted and increases the molecule's internal energy level. Likewise, when an excited molecule releases energy, it can do so in the form of a photon. Depending on the energy of the photon, this could correspond to a change in vibrational, electronic, or rotationalenergy levels.The changes between these levels are called "transitions" and are plotted on the Jablonski diagram.
Radiative transitions involve either the absorption or emission of a photon. As mentioned above, these transitions are denoted with solid arrows with their tails at the initial energy level and their tips at the final energy level.
Nonradiative transitions arise through several different mechanisms, all differently labeled in the diagram. Relaxation of the excited state to its lowest vibrational level is calledvibrational relaxation.This process involves the dissipation of energy from the molecule to its surroundings, and thus it cannot occur for isolated molecules.
A second type of nonradiative transition isinternal conversion(IC), which occurs when a vibrational state of an electronically excited state can couple to a vibrational state of a lower electronic state. The molecule could then subsequently relax further through vibrational relaxation.[4]
A third type isintersystem crossing(ISC); this is a transition to a state with a different spin multiplicity. In molecules with largespin-orbit coupling,intersystem crossing is much more important than in molecules that exhibit only small spin-orbit coupling. ISC can be followed byphosphorescence.
See also[edit]
- Franck–Condon principle
- Grotrian diagram(for atoms)
References[edit]
- ^“Jablonski Diagram.” 2006. In IUPAC Compendium of Chemical Terminoloy, 3rd ed. International Union of Pure and Applied Chemistry.https://doi.org/10.1351/goldbook.J03360.
- ^P., Atkins, P., de Paula, J.Atkins' Physical Chemistry,8th edition (2006), page 494, Oxford University Press.ISBN0-7167-8759-8
- ^Jabłoński, Aleksander "Efficiency of Anti-Stokes Fluorescence in Dyes" Nature 1933, volume 131, pp. 839-840.doi:10.1038/131839b0
- ^Harris, D. C. Lucy, C. A.Quantitative Chemical Analysis,Tenth Edition (2020), pp 457-458, W.H. Freeman and Co.
External links[edit]
- Florida State University: Jablonski diagram primer
- Consequences of Light Absorption – The Jablonski Diagram
