Ketoconazole
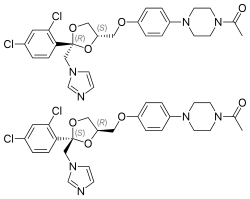 (2R,4S)-(+)-ketoconazole (top) (2S,4R)-(−)-ketoconazole (bottom) | |
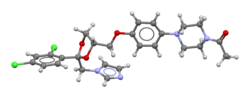 Ball-and-stick modelof (2R,4S)-(+)-ketoconazole | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌkiːtoʊˈkoʊnəˌzoʊl,-zɒl/[1][2] |
| Trade names | Nizoral, others |
| Other names | R-41400; KW-1414 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682816 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth(tablets),topical(cream,shampoo,solution) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokineticdata | |
| Bioavailability | By mouth:37–97%[8] |
| Protein binding | 84 to 99% |
| Metabolism | Extensiveliver(predominantly oxidation,O-dealkylation) |
| Metabolites | N-deacetyl ketoconazole |
| Eliminationhalf-life | Biphasic |
| Excretion | Bile duct(major) andkidney[9] |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.059.680 |
| Chemical and physical data | |
| Formula | C26H28Cl2N4O4 |
| Molar mass | 531.43g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture[9][10] |
| |
| |

Ketoconazole,sold under the brand nameNizoralamong others, is anantiandrogen,antifungal,andantiglucocorticoidmedication used to treat a number offungal infections.[11]Applied to the skin it is used forfungal skin infectionssuch astinea,cutaneous candidiasis,pityriasis versicolor,dandruff,andseborrheic dermatitis.[12]Takenby mouthit is a less preferred option and only recommended for severe infections when other agents cannot be used.[11]Other uses include treatment ofexcessive male-patterned hair growth in womenandCushing's syndrome.[11]
Commonside effectswhenapplied to the skininclude redness.[12]Common side effects when taken by mouth includenausea,headache,andliver problems.[11]Liver problems may result in death or the need for aliver transplantation.[11][13]Other severe side effects when taken by mouth includeQT prolongation,adrenocortical insufficiency,andanaphylaxis.[11][13]It is animidazoleand works by hindering the production ofergosterolrequired for the fungalcell membrane,thereby slowing growth.[11]
Ketoconazole was patented in 1977 by Belgian pharmaceutical companyJanssen,and came into medical use in 1981.[14]It is available as ageneric medicationand formulations that are applied to the skin areover the counterin theUnited Kingdom.[12]In 2021, it was the 161st most commonly prescribed medication in the United States, with more than 3million prescriptions.[15][16]The formulation that is taken by mouth waswithdrawnin the European Union and in Australia in 2013,[17][18]and in China in 2015.[19]In addition, its use was restricted in the United States and Canada in 2013.[18]
Medical uses
[edit]Topical antifungal
[edit]Topically administered ketoconazole is usually prescribed for fungal infections of the skin and mucous membranes, such asathlete's foot,ringworm,candidiasis(yeast infection or thrush),jock itch,andtinea versicolor.[20]Topical ketoconazole is also used as a treatment fordandruff(seborrheic dermatitis of the scalp) and forseborrheic dermatitison other areas of the body, perhaps acting in these conditions by suppressing levels of the fungusMalassezia furfuron the skin.[20][21][22]
Systemic antifungal
[edit]Ketoconazole has activity against many kinds of fungi that may cause human disease, such asCandida,Histoplasma,Coccidioides,andBlastomyces(although it is not active againstAspergillus),chromomycosisandparacoccidioidomycosis.[23][13]First made in 1977,[20]ketoconazole was the first orally-activeazoleantifungal medication.[23]However, ketoconazole has largely been replaced as a first-line systemic antifungal medication by otherazole antifungalagents, such asfluconazoleand/oritraconazole,because of ketoconazole's greater toxicity, poorer absorption, and more limited spectrum of activity.[23][24]
Ketoconazole is used orally in dosages of 200 to 400 mg per day in the treatment of superficial and deep fungal infections.[25]
Off-label uses
[edit]Hair loss
[edit]
Ketoconazole shampoo in conjunction with an oral5α-reductase inhibitorsuch asfinasterideordutasteridehas been used off label to treatandrogenic alopecia.It was speculated that antifungal properties of ketoconazole reduce scalp microflora and consequently may reduce follicular inflammation that contributes to alopecia.[26]
Limited clinical studies suggest ketoconazole shampoo used either alone[27][28]or in combination with other treatments[29]may be useful in reducing hair loss in some cases.[30]
Hormonal
[edit]The side effects of ketoconazole are sometimes harnessed in the treatment of non-fungal conditions. While ketoconazole blocks the synthesis of the sterolergosterolin fungi, in humans, at high dosages (>800 mg/day), it potently inhibits the activity of severalenzymesnecessary for the conversion ofcholesteroltosteroid hormonessuch astestosteroneandcortisol.[23][25]Specifically, ketoconazole has been shown to inhibitcholesterol side-chain cleavage enzyme,which converts cholesterol topregnenolone,17α-hydroxylaseand17,20-lyase,[25]which convertpregnenoloneintoandrogens,and11β-hydroxylase,which converts11-deoxycortisoltocortisol.[31]All of these enzymes are mitochondrialcytochrome p450enzymes.[32]Based on theseantiandrogenandantiglucocorticoideffects, ketoconazole has been used with some success as a second-line treatment for certain forms of advancedprostate cancer[25][33]and for the suppression ofglucocorticoidsynthesis in the treatment ofCushing's syndrome.[34]However, in the treatment of prostate cancer, concomitant glucocorticoid administration is needed to preventadrenal insufficiency.[25]Ketoconazole has additionally been used, in lower dosages, to treathirsutismand, in combination with aGnRH analogue,male-limited precocious puberty.[25]In any case, the risk ofhepatotoxicitywith ketoconazole limits its use in all of these indications, especially in those that are benign such as hirsutism.[25]
Ketoconazole has been used to prevent thetestosteroneflare at the initiation ofGnRH agonisttherapy in men with prostate cancer.[35]
Contraindications
[edit]Oral ketoconazole has variouscontraindications,such as concomitant use with certain other drugs due to knowndrug interactions.[6]Other contraindications of oral ketoconazole includeliver disease,adrenal insufficiency,and knownhypersensitivityto oral ketoconazole.[6]
Side effects
[edit]Gastrointestinal
[edit]Vomiting, diarrhea, nausea, constipation, abdominal pain, upper abdominal pain, dry mouth,dysgeusia,dyspepsia,flatulence,tongue discoloration may occur.[36]
Endocrine
[edit]The drug may causeadrenal insufficiencyso the level of theadrenocortical hormonesshould be monitored while taking it.[13][36]Oral ketoconazole at a dosage range of 400 to 2,000 mg/day has been found to result in a rate ofgynecomastiaof 21%.[37]
Liver
[edit]In July 2013, the USFood and Drug Administration(FDA) issued a warning that taking ketoconazole by mouth can cause severe liver injuries and adrenal gland problems:adrenal insufficiencyand worsening of other related to the gland conditions.[13]It recommends oral tablets should not be a first-line treatment for any fungal infection. It should be used for the treatment of certain fungal infections, known as endemic mycoses, only when alternative antifungal therapies are not available or tolerated.[13]As contraindication it should not be used in people with acute or chronic liver disease.[13]
Hypersensitivity
[edit]Anaphylaxisafter the first dose may occur.[medical citation needed]Other cases of hypersensitivity includeurticaria.[11][6]
Topical formulations
[edit]The topical formulations have not been associated with liver damage, adrenal problems, or drug interactions. These formulations include creams, shampoos, foams, and gels applied to the skin, unlike the ketoconazole tablets, which are taken by mouth.[13]
Pregnancy
[edit]Ketoconazole is categorized aspregnancy category Cin the US.[38]Research in animals has shown it to causeteratogenesiswhen administered in high doses.[38]A subsequent trial in Europe failed to show a risk to infants of mothers receiving ketoconazole.[39]
Overdose
[edit]In the event of anoverdoseof oral ketoconazole, treatment should be supportive and based onsymptoms.[6]Activated charcoalmay be administered within the first hour following overdose of oral ketoconazole.[6]
Interactions
[edit]The concomitant use of the following medications is contraindicated with ketoconazole tablets:[6][36]
- methadone,disopyramide,dronedarone
- irinotecan,lurasidone,colchicine
- alprazolam,oralmidazolam,oraltriazolam
- felodipine,ranolazine,tolvaptan,eplerenone
- HMG-CoA reductase inhibitors:lovastatin,simvastatin
- ergot alkaloids:ergotamine,dihydroergotamine,ergometrine,methylergometrine
- Others:cisapride,nisoldipine,dofetilide,pimozide
And is not recommended:[6][36]
- carbamazepine,phenytoin
- gastric acid suppressants:antacids,antimuscarinics,histamine H2blockers,proton pump inhibitors
- sucralfate
- rifampin,rifabutin,isoniazid
- efavirenz,nevirapine
Ritonaviris known for increasing activity of the ketoconazole so it is recommended to reduce dosage.[6]
There is also a list of drugs which significantly decrease systemic exposure to the ketoconazole and drugs whose systemic exposure is increased by the ketoconazole.[6][36]
Pharmacology
[edit]Pharmacodynamics
[edit]Antifungal activity
[edit]This sectionneeds additional citations forverification.(May 2013) |
As an antifungal, ketoconazole is structurally similar toimidazole,and interferes with the fungal synthesis ofergosterol,a constituent of fungalcell membranes,as well as certainenzymes.As with allazole antifungalagents, ketoconazole works principally by inhibiting theenzymecytochrome P45014α-demethylase(CYP51A1).[32]This enzyme participates in thesterolbiosynthesispathway that leads fromlanosteroltoergosterol.Lower doses offluconazoleanditraconazoleare required to kill fungi compared to ketoconazole, as they have been found to have a greateraffinityfor fungal cell membranes.
Resistance to ketoconazole has been observed in a number of clinical fungal isolates, includingCandida albicans.Experimentally, resistance usually arises as a result of mutations in the sterol biosynthesis pathway. Defects in the sterol 5-6 desaturase enzyme reduce the toxic effects of azole inhibition of the 14-alpha demethylation step.Multidrug-resistance(MDR) genes can also play a role in reducing cellular levels of the drug. As azole antifungals all act at the same point in the sterol pathway, resistant isolates are normally cross-resistant to all members of the azole family.[40][41]
Antihormonal activity
[edit]As anantiandrogen,ketoconazole operates through at least twomechanismsof action. First, and most notably, high oral doses of ketoconazole (e.g. 40 mg three times per day) block both testicular and adrenal androgen biosynthesis, leading to a reduction in circulating testosterone levels.[25][42]It produces this effect through inhibition of17α-hydroxylaseand17,20-lyase,which are involved in the synthesis and degradation of steroids, including theprecursorsoftestosterone.[25]Due to its efficacy at reducing systemic androgen levels, ketoconazole has been used with some success as a treatment for androgen-dependent prostate cancer.[43]Second, ketoconazole is anandrogen receptorantagonist,competing with androgens such as testosterone anddihydrotestosterone(DHT) for binding to theandrogen receptor.This effect is thought to be quite weak however, even with high oral doses of ketoconazole.[44]
Ketoconazole, along withmiconazole,has been found to act as anantagonistof theglucocorticoid receptor.[45][46]
Ketoconazole is aracemic mixtureconsisting ofcis-(2S,4R)-(−) andcis-(2R,4S)-(+) enantiomers.[10]Thecis-(2S,4R) isomer was more potent in inhibitingprogesterone 17α,20-lyasethan its enantiomer (IC50values of 0.05 and 2.38μM, respectively) and in inhibiting11β-hydroxylase(IC50values of 0.152 and 0.608μM, respectively). Both isomers were relatively weak inhibitors of human placentalaromatase.[9]
Oral ketoconazole has been used clinically as a steroidogenesis inhibitor in men, women, and children at dosages of 200 to 1,200 mg/day.[47][48][49]Numerous small studies have investigated the effects of oral ketoconazole on hormone levels in humans.[50]It has been found in men to significantly decrease testosterone and estradiol levels and to significantly increaseluteinizing hormone,progesterone,and17α-hydroxyprogesteronelevels, whereas levels ofandrostenedione,follicle-stimulating hormone,andprolactinwere unaffected.[50][51][48]The ratio of testosterone to estradiol is also decreased during oral ketoconazole therapy in men.[48]Suppression of testosterone levels by ketoconazole is generally partial and has often been found to be transient.[50]Better effects on suppression of testosterone levels have been observed in men when ketoconazole is combined with aGnRH agonistto suppress thehypothalamic–pituitary–gonadal axis,which prevents compensatory upregulation of luteinizing hormonesecretionand consequent activation of gonadal testosterone production.[48]Inpremenopausalwomen withpolycystic ovary syndrome,ketoconazole has been found to significantly decrease levels of androstenedione and testosterone and significantly increase levels of 17α-hydroxyprogesterone and estradiol.[49][52]Studies inpostmenopausalwomen with breast cancer have found that ketoconazole significantly decreases androstenedione levels, slightly decreasesestradiollevels, and does not affectestronelevels.[53]This indicates minimal inhibition of aromatase by ketoconazolein vivoin humans.[53]Ketoconazole has also been found to decrease levels ofendogenouscorticosteroids,such ascortisol,corticosterone,andaldosterone,as well asvitamin D.[54][48]
Ketoconazole has been found to displacedihydrotestosteroneand estradiol fromsex hormone-binding globulinin vitro,but this was not found to be relevantin vivo.[48]
Other activities
[edit]Ketoconazole has been found to inhibit the activity of the cation channelTRPM5.[55]
Pharmacokinetics
[edit]When administered orally, ketoconazole is best absorbed at highlyacidiclevels, soantacidsor other causes of decreasedstomachacid levels will lower the drug's absorption. Absorption can be increased by taking it with an acidic beverage, such ascola.[56]Ketoconazole is verylipophilicand tends to accumulate in fatty tissues.
Chemistry
[edit]Ketoconazole is asyntheticimidazole.[57][58]It is anonsteroidalcompound.[57][58]It is aracemic mixtureof twoenantiomers,levoketoconazole((2S,4R)-(−)-ketoconazole) and dextroketoconazole ((2R,4S)-(+)-ketoconazole).[57][58]Levoketoconazole is under development for potential clinical use as a steroidogenesis inhibitor with bettertolerabilityand lesstoxicitythan ketoconazole. Other steroidogenesis inhibitors besides ketoconazole and levoketoconazole include the nonsteroidal compoundaminoglutethimideand thesteroidalcompoundabiraterone acetate.[citation needed]
History
[edit]Ketoconazole was discovered in 1976 atJanssen Pharmaceuticals.[59]It waspatentedin 1977,[14]followed by introduction in theUnited Statesin July 1981.[18][8][60][14]Following its introduction, ketoconazole was the only systemic antifungal available for almost a decade.[18]Ketoconazole was introduced as the prototypical medication of the imidazole group of antifungals.[61]Oral ketoconazole has been replaced with oralfluconazoleoritraconazolefor manymycoses.[61]
Due to incidence of seriousliver toxicity,the use of oral ketoconazole was suspended in France in July 2011, following review.[18]This event triggered an evaluation of oral ketoconazole throughout the rest of the European Union.[18][62]In 2013, oral ketoconazole was withdrawn in the European Union and Australia, and strict restrictions were placed on the use of oral ketoconazole in the United States and Canada.[18]Oral ketoconazole is indicated for use in these countries when the indication is a severe or life-threatening systemic infection and alternatives are unavailable.[18]However, topical ketoconazole, which does not distribute systemically, is safe and widely used still.[18]
Ketoconazole HRA was approved for use in the European Union for treatment ofCushing's syndromein November 2013.[7][63]
Society and culture
[edit]Generic names
[edit]Ketoconazole is thegeneric nameof the drug and itsINN,USAN,BAN,andJAN.[57][58][64][65]
Brand names
[edit]Ketoconazole has been marketed under a large number of brand names.[57][58][64][65]
Availability
[edit]Ketoconazole is available widely throughout the world.[58][65]
In 2013, theEuropean Medicines Agency'sCommittee for Medicinal Products for Human Use(CHMP) recommended that a ban be imposed on the use of oral ketoconazole for systemic use in humans throughout the European Union, after concluding that the risk of seriousliver injuryfrom systemic ketoconazole outweighs its benefits.[66]
Research
[edit]As of March 2019, orallevoketoconazole(developmental code name COR-003, tentative brand name Recorlev) isphase IIIclinical trialsfor the treatment ofCushing's syndrome.[67]Oral levoketoconazole may have a lower risk of liver toxicity than oral ketoconazole.[68]
Veterinary use
[edit]Ketoconazole is sometimes prescribed as an antifungal byveterinariansfor use in pets, often as unflavored tablets that may need to be cut to smaller size for correct dosage.[69]
References
[edit]- ^"Ketoconazole".Merriam-Webster.com Dictionary.Merriam-Webster.
- ^"Ketoconazole".Dictionary.com Unabridged(Online). n.d.
- ^"FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)".nctr-crs.fda.gov.FDA.Retrieved22 October2023.
- ^"Ketoconazole HRA 200mg Tablets - Summary of Product Characteristics (SmPC)".(emc).18 September 2017.Archivedfrom the original on 2 August 2020.Retrieved1 April2020.
- ^"Ketoconazole 2% w/w Shampoo - Summary of Product Characteristics (SmPC)".(emc).5 October 2015.Archivedfrom the original on 3 August 2020.Retrieved1 April2020.
- ^abcdefghij"Ketoconazole tablet".DailyMed.26 June 2018.Archivedfrom the original on 5 August 2020.Retrieved5 January2020.
- ^ab"Ketoconazole HRA EPAR".European Medicines Agency(EMA). 23 April 2012.Archivedfrom the original on 3 August 2020.Retrieved1 April2020.
- ^abMillikan LE (19 April 2016).Drug Therapy in Dermatology.CRC Press. pp. 82–.ISBN978-0-203-90831-0.
- ^abc"Assessment report: Ketoconazole HRA"(PDF).European Medicines Agency(EMA).Archived(PDF)from the original on 27 August 2016.Retrieved26 August2016.
- ^abArakaki R, Welles B (February 2010). "Ketoconazole enantiomer for the treatment of diabetes mellitus".Expert Opinion on Investigational Drugs.19(2): 185–94.doi:10.1517/13543780903381411.PMID20047506.S2CID26531459.
- ^abcdefgh"Ketoconazole Monograph for Professionals".Drugs.com.American Society of Health-System Pharmacists.Archivedfrom the original on 28 December 2010.Retrieved23 March2019.
- ^abcBritish national formulary: BNF 76(76 ed.). Pharmaceutical Press. 2018. p. 1198.ISBN9780857113382.
- ^abcdefgh"FDA limits usage of Nizoral (ketoconazole) oral tablets due to potentially fatal liver injury and risk of drug interactions and adrenal gland problems".FDA Drug Safety Communication.U.S. Food and Drug Administration.26 July 2013.Archivedfrom the original on 2 December 2013.Retrieved23 November2013.
- ^abcFischer J, Ganellin CR (2006).Analogue-based Drug Discovery.John Wiley & Sons. p. 503.ISBN9783527607495.
- ^"The Top 300 of 2021".ClinCalc.Archivedfrom the original on 15 January 2024.Retrieved14 January2024.
- ^"Ketoconazole - Drug Usage Statistics".ClinCalc.Archivedfrom the original on 8 July 2020.Retrieved14 January2024.
- ^"Oral ketoconazole (Nizoral) 200 mg tablets".Therapeutic Goods Administration (TGA).10 October 2013.Archivedfrom the original on 2 July 2015.Retrieved23 March2019.
- ^abcdefghiGupta AK, Lyons DC (2015). "The Rise and Fall of Oral Ketoconazole".Journal of Cutaneous Medicine and Surgery.19(4): 352–7.doi:10.1177/1203475415574970.PMID25775613.S2CID206695486.
- ^"Quốc gia thực phẩm dược phẩm giam đốc quản lý tổng cục quan vu đình chỉ sinh sản tiêu thụ sử dụng đồng khang tọa khẩu phục chế tề đích công cáo ( 2015 niên đệ 85 hào )"(in Chinese).China Food and Drug Administration.25 June 2015.Archivedfrom the original on 2 July 2015.Retrieved2 July2015.
- ^abcPhillips RM, Rosen T (2013). "Topical Antifungal Agents". In Wolverton SE (ed.).Comprehensive Dermatologic Therapy(3rd ed.). Philadelphia: Saunders. pp. 460–472.ISBN978-1-4377-2003-7.
- ^Neider R, Fritsch PO (2012). "Other Eczematous Eruptions". In Bolognia JL (ed.).Dermatology(3rd ed.). Philadelphia: Saunders. pp. 219–221.ISBN9780723435716.
- ^Young BK, Brodell RT, Cooper KD (2013). "Therapeutic Shampoos". In Wolverton SE (ed.).Comprehensive Dermatologic Therapy(3rd ed.). Philadelphia: Saunders. pp. 562–569.ISBN978-1-4377-2003-7.
- ^abcdFinkel R, Cubeddu LX, Clark MA (2009).Pharmacology(4th ed.). Baltimore: Lippincott Williams & Wilkins. p. 411.
- ^Kauffman CA (2004)."Introduction to the Mycoses".In Goldman L, Ausiello D (eds.).Cecil Textbook of Medicine(22nd ed.). Philadelphia: Saunders. p.2043.ISBN978-0-7216-9652-2.
- ^abcdefghiBecker KL (2001).Principles and Practice of Endocrinology and Metabolism.Lippincott Williams & Wilkins. pp. 1197–.ISBN978-0-7817-1750-2.
- ^McElwee KJ, Shapiro JS (June 2012)."Promising therapies for treating and/or preventing androgenic alopecia".Skin Therapy Letter.17(6): 1–4.PMID22735503.Archivedfrom the original on 12 December 2015.
- ^Piérard-Franchimont C, De Doncker P, Cauwenbergh G, Piérard GE (1998). "Ketoconazole shampoo: effect of long-term use in androgenic alopecia".Dermatology.196(4): 474–7.doi:10.1159/000017954.PMID9669136.S2CID30635892.
- ^Piérard-Franchimont C, Goffin V, Henry F, Uhoda I, Braham C, Piérard GE (October 2002)."Nudging hair shedding by antidandruff shampoos. A comparison of 1% ketoconazole, 1% piroctone olamine and 1% zinc pyrithione formulations".International Journal of Cosmetic Science.24(5): 249–56.doi:10.1046/j.1467-2494.2002.00145.x.hdl:2268/11902.PMID18498517.Archivedfrom the original on 29 August 2021.Retrieved4 July2019.
- ^Khandpur S, Suman M, Reddy BS (August 2002). "Comparative efficacy of various treatment regimens for androgenetic alopecia in men".The Journal of Dermatology.29(8): 489–98.doi:10.1111/j.1346-8138.2002.tb00314.x.PMID12227482.S2CID20886812.
- ^Marks DH, Prasad S, De Souza B, Burns LJ, Senna MM (December 2019). "Topical Antiandrogen Therapies for Androgenetic Alopecia and Acne Vulgaris".Am J Clin Dermatol.21(2): 245–254.doi:10.1007/s40257-019-00493-z.PMID31832993.S2CID209331373.
- ^"MedScape".Ectopic Cortisol Production Derived From Malignant Testicular Masses: Treatment and Management.Nature Publishing Group.Archivedfrom the original on 13 May 2018.Retrieved18 April2015.
- ^abLoose DS, Kan PB, Hirst MA, Marcus RA, Feldman D (May 1983)."Ketoconazole blocks adrenal steroidogenesis by inhibiting cytochrome P450-dependent enzymes".The Journal of Clinical Investigation.71(5): 1495–9.doi:10.1172/JCI110903.PMC437014.PMID6304148.
- ^Zelefsky MJ, Eastham JA, Sartor OA, Kantoff P (2008). DeVita VT, Lawrence TS, Rosenberg SA (eds.).Cancer: Principles & Practice of Oncology(8th ed.). Philadelphia: Lippincott Williams & Wilkins. p. 1443.ISBN9780781772075.
- ^Loli P, Berselli ME, Tagliaferri M (December 1986). "Use of ketoconazole in the treatment of Cushing's syndrome".The Journal of Clinical Endocrinology and Metabolism.63(6): 1365–71.doi:10.1210/jcem-63-6-1365.PMID3023421.
- ^Thompson IM (2001)."Flare Associated with LHRH-Agonist Therapy".Reviews in Urology.3(Suppl 3): S10–4.PMC1476081.PMID16986003.
- ^abcde"Nizoral (Ketoconazole): Side Effects, Interactions, Warning, Dosage & Uses".RxList.Archivedfrom the original on 7 April 2019.Retrieved7 April2019.
- ^Deepinder F, Braunstein GD (September 2012). "Drug-induced gynecomastia: an evidence-based review".Expert Opinion on Drug Safety.11(5): 779–95.doi:10.1517/14740338.2012.712109.PMID22862307.S2CID22938364.
- ^ab"Ketoconazole (Nizoral) Use During Pregnancy".Drugs.com.Archivedfrom the original on 10 April 2020.Retrieved24 May2020.
- ^Kazy Z, Puhó E, Czeizel AE (March 2005)."Population-based case-control study of oral ketoconazole treatment for birth outcomes".Congenital Anomalies.45(1): 5–8.doi:10.1111/j.1741-4520.2005.00053.x.PMID15737124.S2CID41187361.
- ^Cartledge JD, Midgley J, Gazzard BG (December 1997)."Clinically significant azole cross-resistance in Candida isolates from HIV-positive patients with oral candidosis".AIDS.11(15): 1839–44.doi:10.1097/00002030-199715000-00008.PMID9412702.S2CID8440973.
- ^Sanglard D, Ischer F, Monod M, Bille J (February 1997)."Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene".Microbiology.143(Pt 2): 405–16.doi:10.1099/00221287-143-2-405.PMID9043118.
- ^Witjes FJ, Debruyne FM, Fernandez del Moral P, Geboers AD (May 1989). "Ketoconazole high dose in management of hormonally pretreated patients with progressive metastatic prostate cancer. Dutch South-Eastern Urological Cooperative Group".Urology.33(5): 411–5.doi:10.1016/0090-4295(89)90037-X.PMID2652864.
- ^De Coster R, Wouters W, Bruynseels J (January 1996). "P450-dependent enzymes as targets for prostate cancer therapy".The Journal of Steroid Biochemistry and Molecular Biology.56(1–6 Spec No): 133–43.doi:10.1016/0960-0760(95)00230-8.PMID8603034.S2CID42845713.
- ^Eil C (August 1992)."Ketoconazole binds to the human androgen receptor".Hormone and Metabolic Research.24(8): 367–70.doi:10.1055/s-2007-1003337.PMID1526623.S2CID33271618.Archivedfrom the original on 11 February 2020.Retrieved4 July2019.
- ^Loose DS, Stover EP, Feldman D (July 1983)."Ketoconazole binds to glucocorticoid receptors and exhibits glucocorticoid antagonist activity in cultured cells".The Journal of Clinical Investigation.72(1): 404–8.doi:10.1172/jci110982.PMC1129197.PMID6135709.
- ^Duret C, Daujat-Chavanieu M, Pascussi JM, Pichard-Garcia L, Balaguer P, Fabre JM, et al. (July 2006). "Ketoconazole and miconazole are antagonists of the human glucocorticoid receptor: consequences on the expression and function of the constitutive androstane receptor and the pregnane X receptor".Molecular Pharmacology.70(1): 329–39.doi:10.1124/mol.105.022046.PMID16608920.S2CID21455699.
- ^Shaw JC (November 1996). "Antiandrogen therapy in dermatology".International Journal of Dermatology.35(11): 770–8.doi:10.1111/j.1365-4362.1996.tb02970.x.PMID8915726.S2CID39334280.
- ^abcdefSonino N (August 1986). "The endocrine effects of ketoconazole".Journal of Endocrinological Investigation.9(4): 341–7.doi:10.1007/BF03346939.PMID3537102.S2CID9148909.
- ^abWheeler CJ, Keye WR, Peterson CM (2010). "Polycystic Ovary Syndrome".Reproductive Endocrinology and Infertility.Springer. pp. 147–182.doi:10.1007/978-1-4419-1436-1_11.ISBN978-1-4419-1435-4.
- ^abcDrobnis EZ, Nangia AK (2017). "Antimicrobials and Male Reproduction".Impacts of Medications on Male Fertility.Advances in Experimental Medicine and Biology. Vol. 1034. Springer. pp. 131–161.doi:10.1007/978-3-319-69535-8_10.ISBN978-3-319-69534-1.PMID29256130.
- ^Feldman D (November 1986). "Ketoconazole and other imidazole derivatives as inhibitors of steroidogenesis".Endocrine Reviews.7(4): 409–20.doi:10.1210/edrv-7-4-409.PMID3536461.
- ^Gal M, Orly J, Barr I, Algur N, Boldes R, Diamant YZ (May 1994)."Low dose ketoconazole attenuates serum androgen levels in patients with polycystic ovary syndrome and inhibits ovarian steroidogenesis in vitro".Fertility and Sterility.61(5): 823–32.doi:10.1016/S0015-0282(16)56691-6.PMID8174717.
- ^abLønning PE (2009)."New endocrine drugs for treatment of advanced breast cancer".Acta Oncologica.29(3): 379–86.doi:10.3109/02841869009090018.PMID2194539.
- ^Tarbit MH, Robertson WR, Lambert A (1990). "Hepatic and Endocrine Effects of Azole Antifungal Agents".Chemotherapy of Fungal Diseases.Handbook of Experimental Pharmacology. Vol. 96. Springer. pp. 205–229.doi:10.1007/978-3-642-75458-6_10.ISBN978-3-642-75460-9.ISSN0171-2004.
- ^Philippaert K, Kerselaers S, Voets T, Vennekens R (April 2018)."2+-Activated Monovalent Cation-Selective Channels".SLAS Discovery.23(4): 341–352.doi:10.1177/2472555217748932.PMID29316407.
- ^Chin TW, Loeb M, Fong IW (August 1995)."Effects of an acidic beverage (Coca-Cola) on absorption of ketoconazole".Antimicrobial Agents and Chemotherapy.39(8): 1671–5.doi:10.1128/AAC.39.8.1671.PMC162805.PMID7486898.
- ^abcdeElks J (14 November 2014).The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies.Springer. pp. 720–.ISBN978-1-4757-2085-3.Archivedfrom the original on 10 January 2023.Retrieved11 April2017.
- ^abcdefIndex Nominum 2000: International Drug Directory.Taylor & Francis. 2000. pp. 586–.ISBN978-3-88763-075-1.Archivedfrom the original on 10 January 2023.Retrieved22 July2018.
- ^Heeres J, Backx LJ, Mostmans JH, Van Cutsem J (August 1979). "Antimycotic imidazoles. part 4. Synthesis and antifungal activity of ketoconazole, a new potent orally active broad-spectrum antifungal agent".Journal of Medicinal Chemistry.22(8): 1003–5.doi:10.1021/jm00194a023.PMID490531.
- ^William Andrew Publishing (22 October 2013).Pharmaceutical Manufacturing Encyclopedia(3rd ed.). Elsevier. pp. 1997–.ISBN978-0-8155-1856-3.Archivedfrom the original on 10 January 2023.Retrieved14 June2019.
- ^abGolan DE (2008).Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy.Lippincott Williams & Wilkins. pp. 624–.ISBN978-0-7817-8355-2.Archivedfrom the original on 10 January 2023.Retrieved11 April2017.
- ^"Ketoconazole-containing medicines".European Medicines Agency(EMA). 25 July 2013.Archivedfrom the original on 22 February 2024.Retrieved22 February2024.
- ^"Ketoconazole HRA recommended for approval in Cushing's syndrome".European Medicines Agency(EMA). 26 September 2014.Archivedfrom the original on 22 February 2024.Retrieved22 February2024.
- ^abMorton IK, Hall JM (6 December 2012).Concise Dictionary of Pharmacological Agents: Properties and Synonyms.Springer Science & Business Media. pp. 159–.ISBN978-94-011-4439-1.
- ^abc"Ketoconazole".Archivedfrom the original on 23 July 2018.Retrieved22 July2018.
- ^"European Medicines Agency recommends suspension of marketing authorisations for oral ketoconazole"(Press release).European Medicines Agency(EMA). 26 July 2013. Archived fromthe originalon 14 January 2014.
- ^"Levoketoconazole - Strongbridge Biopharma - AdisInsight".Archivedfrom the original on 23 January 2020.Retrieved14 June2019.
- ^Fleseriu M, Castinetti F (December 2016)."Updates on the role of adrenal steroidogenesis inhibitors in Cushing's syndrome: a focus on novel therapies".Pituitary.19(6): 643–653.doi:10.1007/s11102-016-0742-1.PMC5080363.PMID27600150.
- ^KuKanich B (January 2008)."A review of selected systemic antifungal drugs for use in dogs and cats".Veterinary Medicine.Archivedfrom the original on 5 October 2013.
- 7α-Hydroxylase inhibitors
- 11β-Hydroxylase inhibitors
- 21-Hydroxylase inhibitors
- Acetamides
- Antifungals for dermatologic use
- Antiglucocorticoids
- Aromatase inhibitors
- Belgian inventions
- Chloroarenes
- Cholesterol side-chain cleavage enzyme inhibitors
- CYP2D6 inhibitors
- CYP3A4 inhibitors
- CYP17A1 inhibitors
- Dioxolanes
- Disulfiram-like drugs
- Drugs developed by Johnson & Johnson
- Endocrine disruptors
- General cytochrome P450 inhibitors
- Hair loss medications
- Hepatotoxins
- HERG blocker
- Hormonal antineoplastic drugs
- Imidazole antifungals
- Janssen Pharmaceutica
- Lanosterol 14α-demethylase inhibitors
- Nonsteroidal antiandrogens
- Phenylethanolamine ethers
- Piperazines
- Pregnane X receptor antagonists
