Kinase

Inbiochemistry,akinase(/ˈkaɪneɪs,ˈkɪneɪs,-eɪz/)[2]is anenzymethatcatalyzesthe transfer ofphosphategroups fromhigh-energy,phosphate-donating molecules to specificsubstrates.This process is known asphosphorylation,where the high-energyATPmolecule donates a phosphate group to thesubstratemolecule. As a result,kinaseproduces a phosphorylated substrate andADP.Conversely, it is referred to asdephosphorylationwhen the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group (producing a dephosphorylated substrate and the high energy molecule of ATP). These two processes, phosphorylation and dephosphorylation, occur four times duringglycolysis.[3][4][5]
Kinases are part of the larger family ofphosphotransferases.Kinases should not be confused withphosphorylases,which catalyze the addition of inorganic phosphate groups to an acceptor, nor withphosphatases,which remove phosphate groups (dephosphorylation). The phosphorylation state of a molecule, whether it be aprotein,lipidorcarbohydrate,can affect its activity, reactivity and its ability to bind other molecules. Therefore, kinases are critical inmetabolism,cell signalling,protein regulation,cellular transport,secretory processesand many other cellular pathways, which makes them very important to physiology.
Biochemistry and functional relevance[edit]
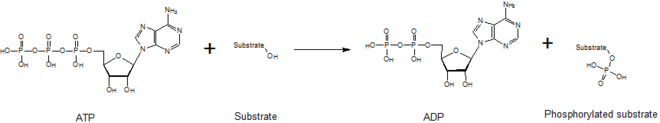
Kinases mediate the transfer of a phosphate moiety from a high energy molecule (such asATP) to their substrate molecule, as seen in the figure below. Kinases are needed to stabilize this reaction because thephosphoanhydridebond contains a high level of energy. Kinases properly orient their substrate and the phosphoryl group within their active sites, which increases the rate of the reaction. Additionally, they commonly use positively chargedamino acidresidues, which electrostatically stabilize the transition state by interacting with the negatively charged phosphate groups. Alternatively, some kinases utilize bound metal cofactors in their active sites to coordinate the phosphate groups. Protein kinases can be classed as catalytically active (canonical) or aspseudokinases,reflecting the evolutionary loss of one or more of the catalytic amino acids that position or hydrolyse ATP.[6]However, in terms of signalling outputs and disease relevance, both kinases and pseudokinases are important signalling modulators in human cells, making kinases important drug targets.[7]
Kinases are used extensively totransmit signalsand regulate complex processes in cells. Phosphorylation of molecules can enhance or inhibit their activity and modulate their ability to interact with other molecules. The addition and removal of phosphoryl groups provides the cell with a means of control because various kinases can respond to different conditions or signals. Mutations in kinases that lead to a loss-of-function or gain-of-function can cause cancer[8]and disease in humans, including certain types ofleukemiaandneuroblastomas,glioblastoma,[9]spinocerebellar ataxia(type 14), forms ofagammaglobulinaemia,and many others.[10]
History and classification[edit]
The first protein to be recognized as catalyzing the phosphorylation of another protein using ATP was observed in 1954 byEugene P. Kennedyat which time he described a liver enzyme that catalyzed the phosphorylation of casein.[citation needed]In 1956,Edmond H. FischerandEdwin G. Krebsdiscovered that the interconversion between phosphorylase a and phosphorylase b was mediated by phosphorylation and dephosphorylation.[11]The kinase that transferred a phosphoryl group to Phosphorylase b, converting it to Phosphorylase a, was named Phosphorylase Kinase. Years later, the first example of a kinase cascade was identified, whereby Protein Kinase A (PKA) phosphorylates Phosphorylase Kinase. At the same time, it was found that PKA inhibitsglycogen synthase,which was the first example of a phosphorylation event that resulted in inhibition. In 1969, Lester Reed discovered thatpyruvate dehydrogenasewas inactivated by phosphorylation, and this discovery was the first clue that phosphorylation might serve as a means of regulation in other metabolic pathways besidesglycogenmetabolism. In the same year, Tom Langan discovered that PKA phosphorylates histone H1, which suggested phosphorylation might regulate nonenzymatic proteins. The 1970s included the discovery ofcalmodulin-dependent protein kinasesand the finding that proteins can be phosphorylated on more than one amino acid residue. The 1990s may be described as the "decade of protein kinase cascades". During this time, theMAPK/ERK pathway,theJAK kinases(a family of protein tyrosine kinases), and the PIP3-dependent kinase cascade were discovered.[12]
Kinases are classified into broad groups by the substrate they act upon: protein kinases, lipid kinases, carbohydrate kinases. Kinases can be found in a variety of species, from bacteria to mold to worms to mammals.[13]More than five hundred different kinases have been identified in humans.[3]Their diversity and their role in signaling makes them an interesting object of study. Various other kinases act on small molecules such aslipids,carbohydrates,amino acids,andnucleotides,either for signaling or to prime them for metabolic pathways. Specific kinases are often named after their substrates. Protein kinases often have multiple substrates, and proteins can serve as substrates for more than one specific kinase. For this reason protein kinases are named based on what regulates their activity (i.e. Calmodulin-dependent protein kinases). Sometimes they are further subdivided into categories because there are several isoenzymatic forms. For example, type I and type II cyclic-AMP dependent protein kinases have identical catalytic subunits but different regulatory subunits that bind cyclic AMP.[14]
Protein kinases[edit]
Protein kinases act on proteins, by phosphorylating them on their serine, threonine, tyrosine, or histidine residues. Phosphorylation can modify the function of a protein in many ways. It can increase or decrease a protein's activity, stabilize it or mark it for destruction, localize it within a specific cellular compartment, and it can initiate or disrupt its interaction with other proteins. The protein kinases make up the majority of all kinases and are widely studied.[15]These kinases, in conjunction withphosphatases,play a major role in protein andenzymeregulation as well as signalling in the cell.
A common point of confusion arises when thinking about the different ways a cell achieves biological regulation. There are countless examples of covalent modifications that cellular proteins can undergo; however, phosphorylation is one of the few reversible covalent modifications. This provided the rationale that phosphorylation of proteins is regulatory. The potential to regulate protein function is enormous given that there are many ways to covalently modify a protein in addition to regulation provided by allosteric control. In his Hopkins Memorial Lecture,Edwin Krebsasserted that allosteric control evolved to respond to signals arising from inside the cell, whereas phosphorylation evolved to respond to signals outside of the cell. This idea is consistent with the fact that phosphorylation of proteins occurs much more frequently ineukaryotic cellsin comparison toprokaryotic cellsbecause the more complex cell type evolved to respond to a wider array of signals.[14]
Cyclin dependent kinases[edit]
Cyclin dependent kinases(CDKs) are a group of several different kinases involved in regulation of thecell cycle.They phosphorylate other proteins on their serine or threonine residues, but CDKs must first bind to acyclinprotein in order to be active.[16]Different combinations of specific CDKs and cyclins mark different parts of the cell cycle. Additionally, the phosphorylation state of CDKs is also critical to their activity, as they are subject to regulation by other kinases (such asCDK-activating kinase) andphosphatases(such asCdc25).[17]Once the CDKs are active, they phosphorylate other proteins to change their activity, which leads to events necessary for the next stage of the cell cycle. While they are most known for their function in cell cycle control, CDKs also have roles in transcription, metabolism, and other cellular events.[18]
Because of their key role in the controlling cell division, mutations in CDKs are often found in cancerous cells. These mutations lead to uncontrolled growth of the cells, where they are rapidly going through the whole cell cycle repeatedly.[19]CDK mutations can be found inlymphomas,breast cancer,pancreatictumors,andlung cancer.Therefore,inhibitors of CDKhave been developed as treatments for some types of cancer.[19]
Mitogen-activated protein kinases[edit]
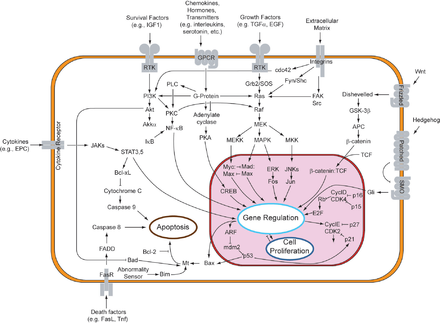
MAP kinases(MAPKs) are a family of serine/threonine kinases that respond to a variety of extracellular growth signals. For example, growth hormone, epidermal growth factor, platelet-derived growth factor, and insulin are all considered mitogenic stimuli that can engage the MAPK pathway. Activation of this pathway at the level of the receptor initiates a signaling cascade whereby theRas GTPaseexchangesGDPforGTP.Next, Ras activatesRaf kinase(also known as MAPKKK), which activatesMEK(MAPKK). MEK activatesMAPK(also known as ERK), which can go on to regulatetranscriptionandtranslation.Whereas RAF and MAPK are both serine/threonine kinases, MAPKK is a tyrosine/threonine kinase.
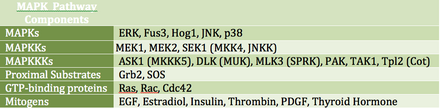
MAPK can regulate transcription factors directly or indirectly. Its major transcriptional targets include ATF-2, Chop, c-Jun, c-Myc, DPC4, Elk-1, Ets1, Max, MEF2C, NFAT4, Sap1a, STATs, Tal, p53, CREB, and Myc. MAPK can also regulate translation by phosphorylating the S6 kinase in the large ribosomal subunit. It can also phosphorylate components in the upstream portion of the MAPK signalling cascade including Ras, Sos, and theEGF receptoritself.[20]
The carcinogenic potential of the MAPK pathway makes it clinically significant. It is implicated in cell processes that can lead to uncontrolled growth and subsequent tumor formation. Mutations within this pathway alter its regulatory effects oncell differentiation,proliferation, survival, andapoptosis,all of which are implicated in various forms ofcancer.[20]
Lipid kinases[edit]
Lipid kinases phosphorylate lipids in the cell, both on the plasma membrane as well as on the membranes of the organelles. The addition of phosphate groups can change the reactivity and localization of the lipid and can be used in signal transmission.
Phosphatidylinositol kinases[edit]

Phosphatidylinositol kinases phosphorylatephosphatidylinositolspecies, to create species such asphosphatidylinositol 3,4-bisphosphate(PI(3,4)P2),phosphatidylinositol 3,4,5-trisphosphate(PIP3), andphosphatidylinositol 3-phosphate(PI3P). The kinases includephosphoinositide 3-kinase(PI3K),phosphatidylinositol-4-phosphate 3-kinase,andphosphatidylinositol-4,5-bisphosphate 3-kinase.The phosphorylation state of phosphatidylinositol plays a major role incellular signalling,such as in the insulin signalling pathway, and also has roles inendocytosis,exocytosisand other trafficking events.[21][22]Mutations in these kinases, such as PI3K, can lead tocancerorinsulin resistance.[23]
The kinase enzymes increase the rate of the reactions by making the inositol hydroxyl group more nucleophilic, often using the side chain of an amino acid residue to act as a general base anddeprotonatethe hydroxyl, as seen in the mechanism below.[24]Here, a reaction betweenadenosine triphosphate (ATP)and phosphatidylinositol is coordinated. The end result is a phosphatidylinositol-3-phosphate as well asadenosine diphosphate (ADP).The enzymes can also help to properly orient the ATP molecule, as well as the inositol group, to make the reaction proceed faster. Metal ions are often coordinated for this purpose.[24]

Sphingosine kinases[edit]
Sphingosine kinase (SK) is a lipid kinase that catalyzes the conversion ofsphingosinetosphingosine-1-phosphate(S1P). Sphingolipids are ubiquitous membrane lipids. Upon activation, sphingosine kinase migrates from the cytosol to the plasma membrane where it transfers a γ phosphate (which is the last or terminal phosphate) fromATPorGTPto sphingosine. The S1P receptor is aGPCRreceptor, so S1P has the ability to regulate G protein signaling. The resulting signal can activate intracellular effectors like ERKs,Rho GTPase,Rac GTPase,PLC,and AKT/PI3K. It can also exert its effect on target molecules inside the cell. S1P has been shown to directly inhibit the histone deacetylase activity ofHDACs.In contrast, the dephosphorylated sphingosine promotes cellapoptosis,and it is therefore critical to understand the regulation of SKs because of its role in determining cell fate. Past research shows that SKs may sustain cancer cell growth because they promote cellular-proliferation, and SK1 (a specific type of SK) is present at higher concentrations in certain types of cancers.
There are two kinases present in mammalian cells, SK1 and SK2. SK1 is more specific compared to SK2, and their expression patterns differ as well. SK1 is expressed in lung, spleen, and leukocyte cells, whereas SK2 is expressed in kidney and liver cells. The involvement of these two kinases in cell survival, proliferation, differentiation, andinflammationmakes them viable candidates forchemotherapeutic therapies.[25]
Carbohydrate kinases[edit]
For many mammals, carbohydrates provide a large portion of the dailycaloricrequirement. To harvest energy fromoligosaccharides,they must first be broken down intomonosaccharidesso they can entermetabolism.Kinases play an important role in almost all metabolic pathways. The figure on the left shows the second phase ofglycolysis,which contains two important reactions catalyzed by kinases. Theanhydridelinkage in 1,3 bisphosphoglycerate is unstable and has a high energy. 1,3-bisphosphogylcerate kinase requires ADP to carry out its reaction yielding 3-phosphoglycerate and ATP. In the final step of glycolysis, pyruvate kinase transfers a phosphoryl group fromphosphoenolpyruvateto ADP, generating ATP and pyruvate.
Hexokinaseis the most common enzyme that makes use of glucose when it first enters the cell. It converts D-glucose to glucose-6-phosphate by transferring the gamma phosphate of an ATP to the C6 position. This is an important step in glycolysis because it traps glucose inside the cell due to the negative charge. In its dephosphorylated form, glucose can move back and forth across the membrane very easily.[26]Mutations in the hexokinase gene can lead to ahexokinase deficiencywhich can cause nonspherocytic hemolyticanemia.[27]
Phosphofructokinase,or PFK, catalyzes the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate and is an important point in the regulation of glycolysis. High levels of ATP, H+,andcitrateinhibit PFK. If citrate levels are high, it means that glycolysis is functioning at an optimal rate. High levels ofAMPstimulate PFK.Tarui's disease,a glycogen storage disease that leads to exercise intolerance, is due to a mutation in the PFK gene that reduces its activity.[28]
Other kinases[edit]
Kinases act upon many other molecules besides proteins, lipids, and carbohydrates. There are many that act on nucleotides (DNA and RNA) including those involved in nucleotide interconverstion, such asnucleoside-phosphate kinasesandnucleoside-diphosphate kinases.[30]Other small molecules that are substrates of kinases includecreatine,phosphoglycerate,riboflavin,dihydroxyacetone,shikimate,and many others.
Riboflavin kinase[edit]
Riboflavin kinase catalyzes the phosphorylation ofriboflavinto createflavin mononucleotide(FMN). It has an ordered binding mechanism where riboflavin must bind to the kinase before it binds to the ATP molecule.[31]Divalentcationshelp coordinate thenucleotide.[31]The general mechanism is shown in the figure below.

Riboflavin kinase plays an important role in cells, asFMNis an importantcofactor.FMNalso is a precursor toflavin adenine dinucleotide(FAD), aredox cofactorused by many enzymes, including many inmetabolism.In fact, there are some enzymes that are capable of carrying out both the phosphorylation of riboflavin toFMN,as well as theFMNtoFADreaction.[32]Riboflavin kinase may help prevent stroke, and could possibly be used as a treatment in the future.[33]It is also implicated in infection, when studied in mice.[34]
Thymidine kinase[edit]
Thymidine kinaseis one of the many nucleoside kinases that are responsible for nucleoside phosphorylation. It phosphorylatesthymidineto createthymidine monophosphate(dTMP). This kinase uses an ATP molecule to supply thephosphateto thymidine, as shown below. This transfer of a phosphate from one nucleotide to another by thymidine kinase, as well as other nucleoside and nucleotide kinases, functions to help control the level of each of the different nucleotides.
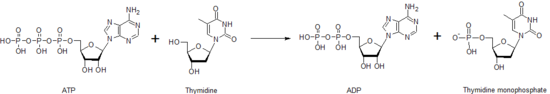
After creation of the dTMP molecule, another kinase,thymidylate kinase,can act upon dTMP to create thediphosphateform, dTDP.Nucleoside diphosphate kinasecatalyzes production ofthymidine triphosphate,dTTP, which is used inDNA synthesis.Because of this, thymidine kinase activity is closely correlated with thecell cycleand used as atumor markerinclinical chemistry.[35]Therefore, it can sometime be used to predict patient prognosis.[36]Patients with mutations in the thymidine kinasegenemay have a certain type ofmitochondrial DNAdepletionsyndrome,a disease that leads to death in early childhood.[37]
See also[edit]
- Activation loop
- Autophosphorylation
- Ca2+/calmodulin-dependent protein kinase
- Cell signaling
- Cyclin-dependent kinase
- G protein-coupled receptor
- Nucleoside-diphosphate kinase
- Phosphatase
- Phosphatidylinositol phosphate kinases
- Phospholipid
- Phosphoprotein
- Phosphorylation
- Phosphotransferase
- Signal transduction
- Thymidine kinase
- Thymidine kinase in clinical chemistry
- Thymidylate kinase
- Wall-associated kinase
References[edit]
- ^Siebold C, Arnold I, Garcia-Alles LF, Baumann U, HErnia B (November 2003)."Crystal structure of the Citrobacter freundii dihydroxyacetone kinase reveals an eight-stranded alpha-helical barrel AKTP-binding domain".The Journal of Biological Chemistry.278(48): 48236–48244.doi:10.1074/jbc.M305942200.PMID12966101.
- ^"kinase".Dictionary.com Unabridged(Online). n.d.Retrieved2022-06-18.
- ^abManning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (December 2002). "The protein kinase complement of the human genome".Science.298(5600): 1912–1934.Bibcode:2002Sci...298.1912M.doi:10.1126/science.1075762.PMID12471243.S2CID26554314.
- ^"Kinase".TheFreeDictionary.com
- ^"History of ATP research milestones from an ATP-related chemistry".Nobelprize.org.
- ^Reiterer V, Eyers PA, Farhan H (September 2014). "Day of the dead: pseudokinases and pseudophosphatases in physiology and disease".Trends in Cell Biology.24(9): 489–505.doi:10.1016/j.tcb.2014.03.008.PMID24818526.
- ^Foulkes DM, Byrne DP and Eyers PA (2017) Pseudokinases: update on their functions and evaluation as new drug targets. Future Med Chem. 9(2):245-265
- ^Samarasinghe B."Hallmarks of Cancer 1".Scientific American.
- ^Bleeker FE, Lamba S, Zanon C, Molenaar RJ, Hulsebos TJ, Troost D, et al. (September 2014)."Mutational profiling of kinases in glioblastoma".BMC Cancer.14:718.doi:10.1186/1471-2407-14-718.PMC4192443.PMID25256166.
- ^Lahiry P, Torkamani A, Schork NJ, Hegele RA (January 2010). "Kinase mutations in human disease: interpreting genotype-phenotype relationships".Nature Reviews. Genetics.11(1): 60–74.doi:10.1038/nrg2707.PMID20019687.S2CID37398118.
- ^Krebs EG (July 1983)."Historical perspectives on protein phosphorylation and a classification system for protein kinases".Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences.302(1108): 3–11.Bibcode:1983RSPTB.302....3K.doi:10.1098/rstb.1983.0033.PMID6137005.
- ^Corbellino M, Poirel L, Aubin JT, Paulli M, Magrini U, Bestetti G, et al. (June 1996)."The role of human herpesvirus 8 and Epstein-Barr virus in the pathogenesis of giant lymph node hyperplasia (Castleman's disease)".Clinical Infectious Diseases.22(6): 1120–1121.doi:10.1093/clinids/22.6.1120.PMID8783733.
- ^Scheeff ED, Bourne PE (October 2005)."Structural evolution of the protein kinase-like superfamily".PLOS Computational Biology.1(5): e49.Bibcode:2005PLSCB...1...49S.doi:10.1371/journal.pcbi.0010049.PMC1261164.PMID16244704.
- ^abKrebs EG (October 1985). "The phosphorylation of proteins: a major mechanism for biological regulation. Fourteenth Sir Frederick Gowland Hopkins memorial lecture".Biochemical Society Transactions.13(5): 813–820.doi:10.1042/bst0130813.PMID2998902.
- ^Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (December 2002). "The protein kinase complement of the human genome".Science.298(5600): 1912–1934.Bibcode:2002Sci...298.1912M.doi:10.1126/science.1075762.PMID12471243.S2CID26554314.
- ^Harper JW, Adams PD (August 2001). "Cyclin-dependent kinases".Chemical Reviews.101(8): 2511–2526.doi:10.1021/cr0001030.PMID11749386.
- ^Karp G (2010).Cell and molecular biology: concepts and experiments(6th ed.). Hoboken, NJ: John Wiley.ISBN9780470483374.
- ^Lim S, Kaldis P (August 2013)."Cdks, cyclins and CKIs: roles beyond cell cycle regulation".Development.140(15): 3079–3093.doi:10.1242/dev.091744.PMID23861057.
- ^abCanavese M, Santo L, Raje N (May 2012)."Cyclin dependent kinases in cancer: potential for therapeutic intervention".Cancer Biology & Therapy.13(7): 451–457.doi:10.4161/cbt.19589.PMID22361734.
- ^abGarrington TP, Johnson GL (April 1999). "Organization and regulation of mitogen-activated protein kinase signaling pathways".Current Opinion in Cell Biology.11(2): 211–218.doi:10.1016/s0955-0674(99)80028-3.PMID10209154.
- ^Sun Y, Thapa N, Hedman AC, Anderson RA (June 2013)."Phosphatidylinositol 4,5-bisphosphate: targeted production and signaling".BioEssays.35(6): 513–522.doi:10.1002/bies.201200171.PMC3882169.PMID23575577.
- ^Heath CM, Stahl PD, Barbieri MA (July 2003). "Lipid kinases play crucial and multiple roles in membrane trafficking and signaling".Histology and Histopathology.18(3): 989–998.doi:10.14670/HH-18.989.PMID12792909.
- ^Cantley LC (2012)."PI 3-kinase and disease".BMC Proceedings.6(Suppl 3): O2.doi:10.1186/1753-6561-6-S3-O2.PMC3395034.
- ^abcMiller S, Tavshanjian B, Oleksy A, Perisic O, Houseman BT, Shokat KM, Williams RL (March 2010)."Shaping development of autophagy inhibitors with the structure of the lipid kinase Vps34".Science.327(5973): 1638–1642.Bibcode:2010Sci...327.1638M.doi:10.1126/science.1184429.PMC2860105.PMID20339072.
- ^Neubauer HA, Pitson SM (November 2013)."Roles, regulation and inhibitors of sphingosine kinase 2".The FEBS Journal.280(21): 5317–5336.doi:10.1111/febs.12314.PMID23638983.
- ^Holzer H, Duntze W (1971). "Metabolic regulation by chemical modification of enzymes".Annual Review of Biochemistry.40:345–374.doi:10.1146/annurev.bi.40.070171.002021.PMID4399446.
- ^"Nonspherocytic hemolytic anemia due to hexokinase deficiency".Archived fromthe originalon 2015-09-05.Retrieved2014-02-24.
- ^"Phosphofructokinase Deficiency Glycogen Storage Disease".
- ^Bauer S, Kemter K, Bacher A, Huber R, Fischer M, Steinbacher S (March 2003). "Crystal structure of Schizosaccharomyces pombe riboflavin kinase reveals a novel ATP and riboflavin-binding fold".Journal of Molecular Biology.326(5): 1463–1473.doi:10.1016/s0022-2836(03)00059-7.PMID12595258.
- ^Voet D, Voet JC, Pratt CW (2008).Fundamentals of biochemistry: life at the molecular level(3rd ed.). Hoboken, NJ: Wiley.ISBN9780470129302.
- ^abKarthikeyan S, Zhou Q, Osterman AL, Zhang H (November 2003). "Ligand binding-induced conformational changes in riboflavin kinase: structural basis for the ordered mechanism".Biochemistry.42(43): 12532–12538.doi:10.1021/bi035450t.PMID14580199.
- ^Galluccio M, Brizio C, Torchetti EM, Ferranti P, Gianazza E, Indiveri C, Barile M (March 2007). "Over-expression in Escherichia coli, purification and characterization of isoform 2 of human FAD synthetase".Protein Expression and Purification.52(1): 175–181.doi:10.1016/j.pep.2006.09.002.PMID17049878.
- ^Zou YX, Zhang XH, Su FY, Liu X (October 2012)."Importance of riboflavin kinase in the pathogenesis of stroke".CNS Neuroscience & Therapeutics.18(10): 834–840.doi:10.1111/j.1755-5949.2012.00379.x.PMC6493343.PMID22925047.
- ^Brijlal S, Lakshmi AV, Bamji MS, Suresh P (September 1996)."Flavin metabolism during respiratory infection in mice".The British Journal of Nutrition.76(3): 453–462.doi:10.1079/BJN19960050.PMID8881717.
- ^Aufderklamm S, Todenhöfer T, Gakis G, Kruck S, Hennenlotter J, Stenzl A, Schwentner C (March 2012). "Thymidine kinase and cancer monitoring".Cancer Letters.316(1): 6–10.doi:10.1016/j.canlet.2011.10.025.PMID22068047.
- ^Topolcan O, Holubec L (February 2008). "The role of thymidine kinase in cancer diseases".Expert Opinion on Medical Diagnostics.2(2): 129–141.doi:10.1517/17530059.2.2.129.PMID23485133.
- ^Götz A, Isohanni P, Pihko H, Paetau A, Herva R, Saarenpää-Heikkilä O, et al. (November 2008)."Thymidine kinase 2 defects can cause multi-tissue mtDNA depletion syndrome".Brain.131(Pt 11): 2841–2850.doi:10.1093/brain/awn236.PMID18819985.

