Low-density lipoprotein

Low-density lipoprotein(LDL) is one of the five major groups oflipoproteinthat transport allfatmolecules around the body in extracellular water.[1]These groups, from least dense to most dense, arechylomicrons(akaULDLby the overall density naming convention),very low-density lipoprotein(VLDL),intermediate-density lipoprotein(IDL), low-density lipoprotein (LDL) andhigh-density lipoprotein(HDL). LDL delivers fat molecules tocells.LDL has been associated with the progression ofatherosclerosis.
Overview
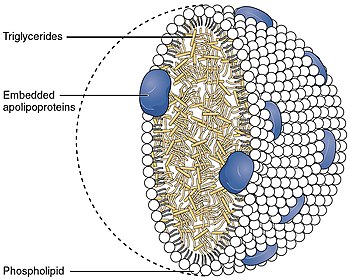
[edit]Lipoproteins transferlipids(fats) around the body in theextracellular fluid,making fats available to body cells forreceptor-mediated endocytosis.[2][3]Lipoproteins are complex particles composed of multipleproteins,typically 80–100 proteins per particle (organized by a singleapolipoprotein Bfor LDL and the larger particles). A single LDL particle is about 220–275angstromsin diameter, typically transporting 3,000 to 6,000 fat molecules per particle, and varying in size according to the number and mix of fat molecules contained within.[4]The lipids carried include all fat molecules withcholesterol,phospholipids,andtriglyceridesdominant; amounts of each vary considerably.[5]
A good clinical interpretation of blood lipid levels is that high LDL, in combination with a high amount of triglycerides, which indicates a high likelihood of the LDL being oxidised, is associated with increased risk ofcardiovascular diseases.[6]
Biochemistry
[edit]Structure
[edit]Each native LDL particle enables emulsification, i.e. surrounding the fatty acids being carried, enabling these fats to move around the body within the water outside cells. Each particle contains a singleapolipoproteinB-100 molecule (Apo B-100,a protein that has 4536amino acidresidues and a mass of 514kDa), along with 80 to 100 additional ancillary proteins. Each LDL has a highly hydrophobic core consisting ofpolyunsaturated fatty acidknown aslinoleateand hundreds to thousands (about 1500 commonly cited as an average) ofesterifiedand unesterified cholesterol molecules. This core also carries varying numbers of triglycerides and other fats and is surrounded by a shell of phospholipids and unesterified cholesterol, as well as the single copy of Apo B-100. LDL particles are approximately 22 nm (0.00000087 in.) to 27.5 nm in diameter and have a mass of about 3 million daltons.[7]Since LDL particles contain a variable and changing number of fatty acid molecules, there is a distribution of LDL particle mass and size.[4]Determining the structure of LDL has been a tough task because of its heterogeneous structure. However, the structure of LDL at human body temperature in native condition, with a resolution of about 16Angstromsusingcryogenic electron microscopy,has been described in 2011.[8]
Physiology
[edit]LDL particles are formed when triglycerides are removed from VLDL by thelipoprotein lipaseenzyme (LPL) and they become smaller and denser (i.e. fewer fat molecules with same protein transport shell), containing a higher proportion of cholesterol esters.[9][10]
Transport into the cell
[edit]When a cell requires additional cholesterol (beyond its current internalHMGCoAproduction pathway), it synthesizes the necessaryLDL receptorsas well asPCSK9,aproprotein convertasethat marks the LDL receptor for degradation.[11]LDL receptors are inserted into the plasma membrane and diffuse freely until they associate withclathrin-coated pits. When LDL receptors bind LDL particles in the bloodstream, the clathrin-coated pits are endocytosed into the cell.
Vesicles containing LDL receptors bound to LDL are delivered to theendosome.In the presence of lowpH,such as that found in the endosome, LDL receptors undergo a conformation change, releasing LDL. LDL is then shipped to thelysosome,wherecholesterol estersin the LDL arehydrolysed.LDL receptors are typically returned to the plasma membrane, where they repeat this cycle. If LDL receptors bind to PCSK9, however, transport of LDL receptors is redirected to the lysosome, where they are degraded.[12]
Role in the innate immune system
[edit]LDL interferes with thequorum sensingsystem that upregulates genes required for invasiveStaphylococcus aureusinfection. The mechanism of antagonism entails binding apolipoprotein B to aS. aureusautoinducerpheromone, preventing signaling through its receptor. Mice deficient in apolipoprotein B are more susceptible to invasive bacterial infection.[13]
LDL size patterns
[edit]LDL can be grouped based on its size: large low density LDL particles are described aspattern A,and small high density LDL particles arepattern B.[14]Pattern Bhas been associated by some with a higher risk forcoronary heart disease.[15]: 1–10 This is thought to be because the smaller particles are more easily able to penetrate theendotheliumofarterial walls.Pattern I,forintermediate,indicates that most LDL particles are very close in size to the normal gaps in the endothelium (26 nm). According to one study, sizes 19.0–20.5 nm were designated as pattern B and LDL sizes 20.6–22 nm were designated as pattern A.[16]Other studies have shown no such correlation at all.[17]
Some evidence suggests the correlation between Pattern B and coronary heart disease is stronger than the correspondence between the LDL number measured in the standard lipid profile test. Tests to measure these LDL subtype patterns have been more expensive and not widely available, so the common lipid profile test is used more often.[15]
There has also been noted a correspondence between higher triglyceride levels and higher levels of smaller, denser LDL particles and alternately lower triglyceride levels and higher levels of the larger, less dense ( "buoyant" ) LDL.[18][19]
With continued research, decreasing cost, greater availability and wider acceptance of otherlipoprotein subclass analysisassay methods, includingNMR spectroscopy,research studies have continued to show a stronger correlation between human clinically obvious cardiovascular events and quantitatively measured particle concentrations.[20]
Oxidized LDL
[edit]Oxidized LDL is a general term for LDL particles with oxidatively modified structural components. As a result, fromfree radicalattack, both lipid and protein parts of LDL can be oxidized in the vascular wall. Besides the oxidative reactions taking place in vascular wall, oxidized lipids in LDL can also be derived from oxidized dietary lipids.[21][22]Oxidized LDL is known to associate with the development ofatherosclerosis,and it is therefore widely studied as a potential risk factor ofcardiovascular diseases.[23]Atherogenicity of oxidized LDL has been explained by lack of recognition of oxidation-modified LDL structures by the LDL receptors, preventing the normal metabolism of LDL particles and leading eventually to development of atherosclerotic plaques.[23]Of the lipid material contained in LDL, various lipid oxidation products are known as the ultimate atherogenic species.[24]Acting as a transporter of these injurious molecules is another mechanism by which LDL can increase the risk of atherosclerosis.[22][25]
Testing
[edit]Blood testscommonly report LDL-C: the amount of cholesterol which is estimated to be contained with LDL particles, on average, using a formula, theFriedewald equation.In clinical context, mathematically calculated estimates of LDL-C are commonly used as an estimate of how much low density lipoproteins are driving progression of atherosclerosis. The problem with this approach is that LDL-C values are commonly discordant with both direct measurements of LDL particles and actual rates of atherosclerosis progression.
Direct LDL measurements are also available and better reveal individual issues but are less often promoted or done due to slightly higher costs and being available from only a couple of laboratories in theUnited States.In 2008, theADAandACCrecognized direct LDL particle measurement by NMR as superior for assessing individual risk of cardiovascular events.[26]
Estimation of LDL particles via cholesterol content
[edit]Chemical measures of lipid concentration have long been the most-used clinical measurement, not because they have the best correlation with individual outcome, but because these lab methods are less expensive and more widely available.
The lipid profile does not measure LDL particles. It only estimates them using the Friedewald equation[19][27] by subtracting the amount of cholesterol associated with other particles, such asHDLand VLDL, assuming a prolonged fasting state, etc.:
- whereHis HDL cholesterol,Lis LDL cholesterol,Cis total cholesterol,Tare triglycerides, and k is 0.20 if the quantities are measured in mg/dL and 0.45 if in mmol/L.
There are limitations to this method, most notably that samples must be obtained after a 12 to 14 h fast and that LDL-C cannot be calculated if plasma triglyceride is >4.52 mmol/L (400 mg/dL). Even at triglyceride levels 2.5 to 4.5 mmol/L, this formula is considered inaccurate.[28]If both total cholesterol and triglyceride levels are elevated then a modified formula, with quantities in mg/dL, may be used
This formula provides an approximation with fair accuracy for most people, assuming the blood was drawn after fasting for about 14 hours or longer, but does not reveal the actual LDL particle concentration because the percentage of fat molecules within the LDL particles which are cholesterol varies, as much as 8:1 variation. There are several formulas published addressing the inaccuracy in LDL-C estimation.[29][30][31]The inaccuracy is based on the assumption that VLDL-C (Very low density lipoprotein cholesterol) is always one-fifth of the triglyceride concentration. Another formulae addresses this issue by using an adjustable factor[32]or by using a regression equation.[33]There are few studies which have compared the LDL-C values derived from this formula and values obtained by direct enzymatic method.[34]Direct enzymatic method are found to be accurate and it has to be the test of choice in clinical situations. In the resource poor settings, the option of using the formula has to be considered.[34]
However, the concentration of LDL particles, and to a lesser extent their size, has a stronger and consistent correlation with individual clinical outcome than the amount of cholesterol within LDL particles, even if the LDL-C estimation is approximately correct. There is increasing evidence and recognition of the value of more targeted and accurate measurements of LDL particles. Specifically, LDL particle number (concentration), and to a lesser extent size, have shown slightly stronger correlations with atherosclerotic progression and cardiovascular events than obtained using chemical measures of the amount of cholesterol carried by the LDL particles.[35]It is possible that the LDL cholesterol concentration can be low, yet LDL particle number high and cardiovascular events rates are high. Correspondingly, it is possible that LDL cholesterol concentration can be relatively high, yet LDL particle number low and cardiovascular events are also low.
Normal ranges
[edit]In the US, theAmerican Heart Association,NIH,andNCEPprovide a set of guidelines for fasting LDL-Cholesterol levels, estimated or measured, and risk for heart disease. As of about 2005, these guidelines were:[36][37][38]
| Levelmg/dL | Levelmmol/L | Interpretation |
|---|---|---|
| 25 to <50 | <1.3 | Optimal LDL cholesterol, levels in healthy young children before onset of atherosclerotic plaque in heart artery walls |
| <70 | <1.8 | Optimal LDL cholesterol, corresponding to lower rates of progression, promoted as a target option for those known to clearly have advanced symptomatic cardiovascular disease |
| <100 | <2.6 | Optimal LDL cholesterol, corresponding to lower, but not zero, rates for symptomatic cardiovascular disease events |
| 100 to 129 | 2.6 to 3.3 | Near optimal LDL level, corresponding to higher rates for developing symptomatic cardiovascular disease events |
| 130 to 159 | 3.3 to 4.1 | Borderline high LDL level, corresponding to even higher rates for developing symptomatic cardiovascular disease events |
| 160 to 199 | 4.1 to 4.9 | High LDL level, corresponding to much higher rates for developing symptomatic cardiovascular disease events |
| >200 | >4.9 | Very high LDL level, corresponding to highest increased rates of symptomatic cardiovascular disease events |
Over time, with more clinical research, these recommended levels keep being reduced because LDL reduction, including to abnormally low levels, was the most effective strategy for reducing cardiovascular death rates in one largedouble blind,randomized clinical trial of men withhypercholesterolemia;[39]far more effective than coronary angioplasty/stenting or bypass surgery.[40]
For instance, for people with known atherosclerosis diseases, the 2004 updated American Heart Association, NIH and NCEP recommendations are for LDL levels to be lowered to less than 70 mg/dL. This low level of less than 70 mg/dL was recommended for primary prevention of 'very-high risk patients' and in secondary prevention as a 'reasonable further reduction'. This position was disputed.[41]Statin drugs involved in such clinical trials havenumerous physiological effectsbeyond simply the reduction of LDL levels.
From longitudinal population studies following progression of atherosclerosis-related behaviors from early childhood into adulthood,[42]the usual LDL in childhood, before the development offatty streaks,is about 35 mg/dL. However, all the above values refer to chemical measures of lipid/cholesterol concentration within LDL, not measured low-density lipoprotein concentrations, the accurate approach.[35]
A study was conducted measuring the effects of guideline changes on LDL cholesterol reporting and control for diabetes visits in the US from 1995 to 2004. It was found that although LDL cholesterol reporting and control for diabetes and coronary heart disease visits improved continuously between 1995 and 2004,[43][44]neither the 1998 ADA guidelines nor the 2001 ATP III guidelines increased LDL cholesterol control for diabetes relative to coronary heart disease.[45]
Direct measurement of LDL particle concentrations
[edit]There are several competing methods for measurement of lipoprotein particle concentrations and size. The evidence is that the NMR methodology (developed, automated & greatly reduced in costs while improving accuracy as pioneered byJim Otvosand associates) results in a 22-25% reduction in cardiovascular events within one year,[46]contrary to the longstanding claims by many in the medical industry that the superiority over existing methods was weak, even by statements of some proponents.[47]
Since the later 1990s, because of the development of NMR measurements, it has been possible to clinically measure lipoprotein particles at lower cost [under $80 US (including shipping) & is decreasing; versus the previous costs of >$400 to >$5,000] and higher accuracy. There are two other assays for LDL particles, however, like LDL-C, most only estimate LDL particle concentrations.
Direct LDL particle measurement by NMR was mentioned by the ADA and ACC, in a 28 March 2008 joint consensus statement,[48]as having advantages for predicting individual risk of atherosclerosis disease events, but the statement noted that the test is less widely available, is more expensive [about $13.00 US (2015 without insurance coverage) from some labs which use the Vantera Analyzer[49]]. Debate continues that it is "...unclear whether LDL particle size measurements add value to measurement of LDL-particle concentration", though outcomes have always tracked LDL particle, not LDL-C, concentrations.
Using NMR, the total LDL particle concentrations, in nmol/L plasma, are typically subdivided by percentiles referenced to the 5,382 men and women, not on any lipid medications, who are participating in the MESA trial.[50]
LDL particle concentration can also be measured by measuring the concentration of the protein ApoB, based on the generally accepted principle that each LDL or VLDL particle carries one ApoB molecule.[51]
Optimal ranges
[edit]The LDL particle concentrations are typically categorized by percentiles, <20%, 20–50%, 50th–80th%, 80th–95% and >95% groups of the people participating and being tracked in theMESA trial,a medical research study sponsored by the United States National Heart, Lung, and Blood Institute.
| MESA Percentile | LDL particles nmol/L | Interpretation |
|---|---|---|
| 0–20% | <1,000 | Those with lowest rate of cardiovascular disease events & low (optimal) LDL particle concentration |
| 20–50% | 1,000–1,299 | Those with moderate rate of cardiovascular disease events & moderate LDL particle concentration |
| 50–80% | 1,300–1,599 | Those with Borderline-High rate of cardiovascular disease events & higher LDL particle concentration |
| 89–95% | 1,600–2,000 | Those with High rate of cardiovascular disease events and even higher LDL particle concentration |
| >95% | >2,000 | Those with very high rate of cardiovascular disease events and highest LDL particle concentration |
The lowest incidence of atherosclerotic events over time occurs within the <20% group, with increased rates for the higher groups. Multiple other measures, including particle sizes, small LDL particle concentrations, large total and HDL particle concentrations, along with estimations ofinsulin resistancepattern and standard cholesterol lipid measurements (for comparison of the plasma data with the estimation methods discussed above) are also routinely provided.
Lowering LDL-cholesterol
[edit]| Markers indicating a need for LDL-C Reduction
(Per 2004 United States Government Minimum Guidelines[52][53]) | ||||
|---|---|---|---|---|
| If the patient's cardiac risk is... | then the patient should consider LDL-C reduction if the count in mg/dL is over... | and LDL-C reduction is indicated if the count in mg/dL is over... | ||
| High, meaning a 20% or greater risk of heart attack within 10 years, or an extreme risk factor | 70[54] | 100[54] | ||
| moderately high, meaning a 10-20% risk of heart attack within 10 years and more than 2 heart attack risk factors | 100[54] | 130[54] | ||
| moderate, meaning a 10% risk of heart attack within 10 years and more than 2 heart attack risk factors | 130[54] | 160[54] | ||
| low, meaning less than 10% risk of heart attack within 10 years and 1 or 0 heart attack risk factors | 160[54] | 190[54] | ||
Themevalonate pathwayserves as the basis for the biosynthesis of many molecules, including cholesterol. The enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG CoA reductase) is an essential component and performs the first of 37 steps within the cholesterol production pathway, and is present in every animal cell.
LDL-C is not a measurement of actual LDL particles. LDL-C is only an estimate (not measured from the individual's blood sample) of how much cholesterol is being transported by all LDL particles, which is either a smaller concentration of large particles or a high concentration of small particles. LDL particles carry many fat molecules (typically 3,000 to 6,000 fat molecules per LDL particle); this includes cholesterol, triglycerides, phospholipids and others. Thus even if the hundreds to thousands of cholesterol molecules within an average LDL particle were measured, this does not reflect the other fat molecules or even the number of LDL particles.
Pharmaceutical
[edit]- PCSK9 inhibitors,in clinical trials, by several companies, are more effective for LDL reduction than the statins, including statins alone at high dose (though not necessarily the combination of statins plus ezetimibe).
- Statinsreduce high levels of LDL particles by inhibiting the enzyme HMG-CoA reductase in cells, the rate-limiting step of cholesterol synthesis. To compensate for the decreased cholesterol availability, synthesis of LDL receptors (including hepatic) is increased, resulting in an increased clearance of LDL particles from the extracellular water, including of the blood.
- Ezetimibereduces intestinal absorption of cholesterol, thus can reduce LDL particle concentrations when combined with statins.[55]
- Niacin(B3), lowers LDL by selectively inhibiting hepatic diacylglycerol acyltransferase 2, reducingtriglyceridesynthesis and VLDL secretion through a receptor HM74[56]and HM74A or GPR109A.[57]
- SeveralCETP inhibitorshave been researched to improve HDL concentrations, but so far, despite dramatically increasing HDL-C, have not had a consistent track record in reducing atherosclerosis disease events. Some have increased mortality rates compared with placebo.
- Clofibrateis effective at lowering cholesterol levels, but has been associated with significantly increased cancer and stroke mortality, despite lowered cholesterol levels.[58]Other developed and tested fibrates, e.g. fenofibric acid[59]have had a better track record and are primarily promoted for lowering VLDL particles (triglycerides), not LDL particles, yet can help some in combination with other strategies.
- Sometocotrienols,especially delta- and gamma-tocotrienols, are being promoted as statin alternative non-prescription agents to treat high cholesterol, having been shown in vitro to have an effect. In particular, gamma-tocotrienol appears to be another HMG-CoA reductase inhibitor, and can reduce cholesterol production.[60]As with statins, this decrease in intra-hepatic (liver) LDL levels may induce hepatic LDL receptor up-regulation, also decreasing plasma LDL levels. As always, a key issue is how benefits and complications of such agents compare with statins—molecular tools that have been analyzed in large numbers of human research and clinical trials since the mid-1970s.
- Phytosterolsare widely recognized as having a proven LDL cholesterol lowering efficacy'[61]A 2018 review found a dose-response relationship for phytosterols, with intakes of 1.5 to 3 g/day lowering LDL-C by 7.5% to 12%,[62]but reviews as of 2017 had found no data indicating that the consumption of phytosterols may reduce the risk of CVD.[63]Current supplemental guidelines for reducing LDL recommend doses of phytosterols in the 1.6-3.0 grams per day range (Health Canada, EFSA, ATP III, FDA) with a 2009 meta-analysis demonstrating an 8.8% reduction in LDL-cholesterol at a mean dose of 2.15 gram per day.[64]
Lifestyle
[edit]LDL cholesterol can be lowered by through dietary intervention by limiting foods withsaturated fatand avoiding foods withtrans fat.[65]Saturated fats are found in meat products (including poultry), full-fat dairy, eggs, and refined tropical oils like coconut and palm.[66]Added trans fat (in the form of partially hydrogenated oils) has been banned in the USA since 2021.[67]However, trans fat can still be found in red meat and dairy products as it is produced in small amounts by ruminants such as sheep and cows.[68]LDL cholesterol can also be lowered by increasing consumption of soluble fiber and plant-based foods.[65]
Another lifestyle approach to reduce LDL cholesterol has been minimizing total body fat, in particular fat stored inside theabdominal cavity(visceral body fat). Visceral fat, which is more metabolically active than subcutaneous fat, has been found to produce many enzymatic signals, e.g.resistin,which increaseinsulin resistanceand circulating VLDL particle concentrations, thus both increasing LDL particle concentrations and accelerating the development of diabetes mellitus.
Research
[edit]Gene editing
[edit]In 2021, scientists demonstrated thatCRISPR gene editingcan decrease blood levels of LDL cholesterol inMacaca fascicularismonkeys for months by 60% viaknockoutofPCSK9in theliver.[69][70]
See also
[edit]- Catechin
- Cholesterol
- Lysosomal acid lipase deficiency
- Cholesteryl ester storage disease
- Coenzyme Q10
- Flavonoid
- Glutathione
- Health effects of tea
- High density lipoprotein
- LDL receptor
- Lipid profile
- Lipoprotein(a)
- Lipoprotein-X
- Melatonin
- Polyphenol antioxidant
- Saturated fat
- Stanol ester
- Sterol ester
- Triglyceride
- Vitamin A
- Vitamin C
- Vitamin E
Notes and references
[edit]- ^"LDL and HDL: Bad and Good Cholesterol".Centers for Disease Control and Prevention.CDC.Retrieved11 September2017.
- ^Dashti M, Kulik W, Hoek F, Veerman EC, Peppelenbosch MP, Rezaee F (2011)."A phospholipidomic analysis of all defined human plasma lipoproteins".Sci. Rep.1(139): 139.Bibcode:2011NatSR...1E.139D.doi:10.1038/srep00139.PMC3216620.PMID22355656.
- ^Dashty M, Motazacker MM, Levels J, de Vries M, Mahmoudi M, Peppelenbosch MP, Rezaee F (2014). "Proteome of human plasma very low-density lipoprotein and low-density lipoprotein exhibits a link with coagulation and lipid metabolism".Thromb. Haemost.111(3): 518–530.doi:10.1160/TH13-02-0178.PMID24500811.S2CID20566238.
- ^abSegrest JP, Jones MK, De Loof H, Dashti N (September 2001)."Structure of apolipoprotein B-100 in low density lipoproteins".Journal of Lipid Research.42(9): 1346–67.doi:10.1016/S0022-2275(20)30267-4.PMID11518754.
- ^Sira, Elevina E. Pérez (2021-10-05).Foods for Special Dietary Regimens.Bentham Science Publishers.ISBN978-981-4998-07-9.
- ^Carson, Jo Ann S.; Lichtenstein, Alice H.; Anderson, Cheryl A.M.; Appel, Lawrence J.; Kris-Etherton, Penny M.; Meyer, Katie A.; Petersen, Kristina; Polonsky, Tamar; Van Horn, Linda (2020-01-21)."Dietary cholesterol and cardiovascular risk: A science advisory from the American Heart Association".Circulation.141(3): e39–e53.doi:10.1161/cir.0000000000000743.ISSN0009-7322.PMID31838890.
- ^Campos, Hannia (1992)."LDL Particle Size Distribution".Arteriosclerosis, Thrombosis, and Vascular Biology.12(12): 1410–1419.doi:10.1161/01.ATV.12.12.1410.PMID1450174.
- ^Kumar V, Butcher SJ, Katrina O, Engelhardt P, Heikkonen J, Kaski K, Ala-Korpela M, Kovanen PT (May 2011)."Three-Dimensional cryoEM Reconstruction of Native LDL Particles to 16Å Resolution at Physiological Body Temperature".PLOS ONE.6(5): e18841.Bibcode:2011PLoSO...618841K.doi:10.1371/journal.pone.0018841.PMC3090388.PMID21573056.
- ^Pirahanchi, Yasaman; Sinawe, Hadeer; Dimri, Manjari (2022),"Biochemistry, LDL Cholesterol",StatPearls,Treasure Island (FL): StatPearls Publishing,PMID30137845,retrieved2022-12-26
- ^Sun, Hung-Yu; Lin, Chun-Chieh; Lee, Jin-Ching; Wang, Shainn-Wei; Cheng, Pin-Nan; Wu, I.-Chin; Chang, Ting-Tsung; Lai, Ming-Derg; Shieh, Dar-Bin; Young, Kung-Chia (July 3, 2013)."Very low-density lipoprotein/lipo-viro particles reverse lipoprotein lipase-mediated inhibition of hepatitis C virus infection via apolipoprotein C-III".Gut.62(8): 1193–1203.doi:10.1136/gutjnl-2011-301798.ISSN1468-3288.PMID22689516.S2CID326884.
- ^Zhang, Da-Wei; Garuti, Rita; Tang, Wan-Jin; Cohen, Jonathan C.; Hobbs, Helen H. (2008-09-02)."Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor".Proceedings of the National Academy of Sciences of the United States of America.105(35): 13045–13050.Bibcode:2008PNAS..10513045Z.doi:10.1073/pnas.0806312105.ISSN0027-8424.PMC2526098.PMID18753623.
- ^Santulli G, Jankauskas SS, Gambardella J (May 2021)."Inclisiran: a new milestone on the PCSK9 road to tackle cardiovascular risk".Eur Heart J Cardiovasc Pharmacother.7(3): e11–e12.doi:10.1093/ehjcvp/pvab014.PMID33655296.
- ^Peterson MM, Mack JL, Hall PR, et al. (December 2008)."Apolipoprotein B Is an innate barrier against invasive Staphylococcus aureus infection".Cell Host & Microbe.4(6): 555–66.doi:10.1016/j.chom.2008.10.001.PMC2639768.PMID19064256.
- ^"When it comes to LDL, size matters".HLTH Code.2022-07-18.Retrieved2022-08-04.
- ^abIvanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN (2017)."Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases".Oxidative Medicine and Cellular Longevity.2017(10): 1273042.doi:10.1155/2017/1273042.PMC5441126.PMID28572872.
- ^Bhalodkar, Narendra C.; Blum, Steve; Rana, Thakor; Kitchappa, Radha; Bhalodkar, Ami N.; Enas, Enas A. (1 May 2005)."Comparison of high-density and low-density lipoprotein cholesterol subclasses and sizes in Asian Indian women with Caucasian women from the framingham offspring study".Clin Cardiol.28(5): 247–251.doi:10.1002/clc.4960280510.PMC6654695.PMID15971461.
- ^"No association between 'bad cholesterol' and elderly deaths: Systematic review of studies of over 68,000 elderly people also raises questions about the benefits of statin drug treatments".sciencedaily.com.
- ^Superko HR, Nejedly M, Garrett B (2002). "Small LDL and its clinical importance as a new CAD risk factor: a female case study".Progress in Cardiovascular Nursing.17(4): 167–73.doi:10.1111/j.0889-7204.2002.01453.x.PMID12417832.
- ^abWarnick GR, Knopp RH, Fitzpatrick V, Branson L (January 1990)."Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints".Clinical Chemistry.36(1): 15–9.doi:10.1093/clinchem/36.1.15.PMID2297909.Archived fromthe originalon 2019-09-12.Retrieved2009-11-02.
- ^Otvos J (June 1999)."Measurement of triglyceride-rich lipoproteins by nuclear magnetic resonance spectroscopy".Clin Cardiol.22(6 Suppl): II21–7.doi:10.1002/clc.4960221405.PMC6655988.PMID10376193.
- ^Staprans, I.; Rapp, J. H.; Pan, X. M.; Feingold, K. R. (1996)."Oxidized lipids in the diet are incorporated by the liver into very low density lipoprotein in rats".Journal of Lipid Research.37(2): 420–30.doi:10.1016/S0022-2275(20)37628-8.PMID9026539.
- ^abAhotupa, Markku (2017)."Oxidized lipoprotein lipids and atherosclerosis".Free Radical Research.51(4): 439–447.doi:10.1080/10715762.2017.1319944.PMID28412863.S2CID3397099.
- ^abStocker, Roland; Keaney, John F. (2004). "Role of Oxidative Modifications in Atherosclerosis".Physiological Reviews.84(4): 1381–1478.doi:10.1152/physrev.00047.2003.PMID15383655.
- ^Birukov, K. G. (2006). "Oxidized lipids: The two faces of vascular inflammation".Current Atherosclerosis Reports.8(3): 223–31.doi:10.1007/s11883-006-0077-x.PMID16640959.S2CID7852910.
- ^Shao, Baohai; Heinecke, Jay W. (2009)."HDL, lipid peroxidation, and atherosclerosis".Journal of Lipid Research.50(4): 599–601.doi:10.1194/jlr.E900001-JLR200.PMC2656652.PMID19141435.
- ^John D. Brunzell, MD, FACP, Michael Davidson, MD, FACC, Curt D. Furberg, MD, PhD, Ronald B. Goldberg, MD, Barbara V. Howard, PhD, James H. Stein, MD, FACC, FACP and Joseph L. Witztum, MD Lipoprotein Management in Patients With Cardiometabolic Risk, J Am Coll Cardiol, 2008; 51:1512-1524.[1]Archived2012-02-27 at theWayback Machine
- ^Friedewald WT, Levy RI, Fredrickson DS (June 1972)."Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge".Clinical Chemistry.18(6): 499–502.doi:10.1093/clinchem/18.6.499.PMID4337382.Archived fromthe originalon 2019-09-12.Retrieved2008-05-05.
- ^Sniderman AD, Blank D, Zakarian R, Bergeron J, Frohlich J (October 2003). "Triglycerides and small dense LDL: the twin Achilles heels of the Friedewald formula".Clinical Biochemistry.36(7): 499–504.doi:10.1016/S0009-9120(03)00117-6.PMID14563441.
- ^Anandaraja, S.; Narang, R.; Godeswar, R.; Laksmy, R.; Talwar, K.K. (June 2005)."Low-density lipoprotein cholesterol estimation by a new formula in Indian population".International Journal of Cardiology.102(1): 117–120.doi:10.1016/j.ijcard.2004.05.009.PMID15939107.
- ^de Cordova, Caio Mauricio Mendes; de Cordova, Mauricio Mendes (January 2013)."A new accurate, simple formula for LDL-cholesterol estimation based on directly measured blood lipids from a large cohort".Annals of Clinical Biochemistry: International Journal of Laboratory Medicine.50(1): 13–19.doi:10.1258/acb.2012.011259.ISSN0004-5632.PMID23108766.S2CID207193749.
- ^Chen, Yunqin; Zhang, Xiaojin; Pan, Baishen; Jin, Xuejuan; Yao, Haili; Chen, Bin; Zou, Yunzeng; Ge, Junbo; Chen, Haozhu (2010)."A modified formula for calculating low-density lipoprotein cholesterol values".Lipids in Health and Disease.9(1): 52.doi:10.1186/1476-511X-9-52.ISSN1476-511X.PMC2890624.PMID20487572.S2CID414939.
- ^Martin, Seth S.; Blaha, Michael J.; Elshazly, Mohamed B.; Toth, Peter P.; Kwiterovich, Peter O.; Blumenthal, Roger S.; Jones, Steven R. (2013-11-20)."Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile".JAMA.310(19): 2061–2068.doi:10.1001/jama.2013.280532.ISSN1538-3598.PMC4226221.PMID24240933.
- ^Sampson, Maureen; Ling, Clarence; Sun, Qian; Harb, Roa; Ashmaig, Mohmed; Warnick, Russell; Sethi, Amar; Fleming, James K.; Otvos, James D.; Meeusen, Jeff W.; Delaney, Sarah R.; Jaffe, Allan S.; Shamburek, Robert; Amar, Marcelo; Remaley, Alan T. (2020-05-01)."A New Equation for Calculation of Low-Density Lipoprotein Cholesterol in Patients With Normolipidemia and/or Hypertriglyceridemia".JAMA Cardiology.5(5): 540–548.doi:10.1001/jamacardio.2020.0013.ISSN2380-6591.PMC7240357.PMID32101259.
- ^abRamasamy, Jagadish; Job, Victoria; Mani, Thenmozhi; Jacob, Molly (2021-05-06)."Calculated values of serum LDL-cholesterol (LDL-C) - for better or worse?".Nutrition, Metabolism, and Cardiovascular Diseases: NMCD.31(5): 1486–1493.doi:10.1016/j.numecd.2021.01.016.ISSN1590-3729.PMID33744036.S2CID232309411.
- ^abSniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, Ference BA (December 2019)."Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review".JAMA Cardiology.4(12): 1287–1295.doi:10.1001/jamacardio.2019.3780.PMC7369156.PMID31642874.
- ^ "Cholesterol Levels".American Heart Association.Retrieved2009-11-14.
- ^ "What Do My Cholesterol Levels Mean?"(PDF).American Heart Association. September 2007.Retrieved2009-11-14.
- ^ "Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Executive Summary"(PDF).National Heart, Lung, and Blood Institute (NHLBI).National Institutes of Health. May 2001.
- ^Shepherd J, Cobbe SM, Ford I, et al. (November 1995)."Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group".The New England Journal of Medicine.333(20): 1301–7.doi:10.1056/NEJM199511163332001.PMID7566020.
- ^William E. Boden; et al. (April 2007)."Optimal Medical Therapy with or without PCI for Stable Coronary Disease".The New England Journal of Medicine.356(15): 1503–1516.doi:10.1056/NEJMoa070829.PMID17387127.
- ^Hayward, Rodney A. (3 October 2006). "Narrative Review: Lack of Evidence for Recommended Low-Density Lipoprotein Treatment Targets: A Solvable Problem".Ann Intern Med.145(7): 520–30.doi:10.7326/0003-4819-145-7-200610030-00010.PMID17015870.S2CID29849069.
- ^Cybulska, Barbara; Kłosiewicz-Latoszek, Longina; Penson, Peter E.; Nabavi, Seyed Mohammad; Lavie, Carl J.; Banach, Maciej; International Lipid Expert Panel (ILEP) (2021)."How much should LDL cholesterol be lowered in secondary prevention? Clinical efficacy and safety in the era of PCSK9 inhibitors".Progress in Cardiovascular Diseases.67:65–74.doi:10.1016/j.pcad.2020.12.008.ISSN1873-1740.PMID33383060.S2CID229942314.
- ^Wolska, Anna; Remaley, Alan T. (2020)."Measuring LDL-cholesterol: what is the best way to do it?".Current Opinion in Cardiology.35(4): 405–411.doi:10.1097/HCO.0000000000000740.ISSN1531-7080.PMC7360339.PMID32412961.
- ^Howard, B. V.; Robbins, D. C.; Sievers, M. L.; Lee, E. T.; Rhoades, D.; Devereux, R. B.; Cowan, L. D.; Gray, R. S.; Welty, T. K.; Go, O. T.; Howard, W. J. (2000-03-01). "LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: The Strong Heart Study".Arteriosclerosis, Thrombosis, and Vascular Biology.20(3): 830–835.doi:10.1161/01.atv.20.3.830.ISSN1079-5642.PMID10712410.
- ^Wang, Y Richard; G Caleb Alexander; David O Meltzer (December 2005)."Lack of Effect of Guideline Changes on LDL Cholesterol Reporting and Control for Diabetes Visits in the U.S., 1995–2004".Diabetes Care.28(12): 2942–2944.doi:10.2337/diacare.28.12.2942.PMID16306559.
- ^Peter P. Toth; Michael Grabner; Rajeshwari S. Punekar; Ralph A. Quimbo; Mark J. Cziraky; Terry A. Jacobson (August 2014)."Cardiovascular risk in patients achieving low-density lipoprotein cholesterol and particle targets".Atherosclerosis.235(2): 585–591.doi:10.1016/j.atherosclerosis.2014.05.914.PMID24956532.
- ^Krauss RM (August 2010). "Lipoprotein subfractions and cardiovascular disease risk".Current Opinion in Lipidology.21(4): 305–11.doi:10.1097/MOL.0b013e32833b7756.PMID20531184.S2CID1775446.
- ^Brunzell, John D.; Davidson, Michael; Furberg, Curt D.; Goldberg, Ronald B.; Howard, Barbara V.; Stein, James H.; Witztum, Joseph L. (15 April 2008). "Lipoprotein Management in Patients With Cardiometabolic Risk".J Am Coll Cardiol.51(15): 1512–1524.doi:10.1016/j.jacc.2008.02.034.PMID18402913.
- ^"Google".www.google.com.
- ^"MESA - Multi-Ethnic Study of Atherosclerosis".www.mesa-nhlbi.org.
- ^Sniderman, A. D.; Thanassoulis, G.; Glavinovic, T.; Navar, A. M.; Pencina, M.; Catapano, A.; Ference, B. A. (1 Dec 2019)."Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review".JAMA Cardiology.4(12): 1287–1295.doi:10.1001/jamacardio.2019.3780.PMC7369156.PMID31642874.
- ^"Management of Blood Cholesterol in Adults: Systematic Evidence Review from the Cholesterol Expert Panel | National Heart, Lung, and Blood Institute (NHLBI)".Archived fromthe originalon 2014-11-25.Retrieved2014-11-16.
- ^"Archived copy"(PDF).Archived fromthe original(PDF)on 2016-03-03.Retrieved2014-11-16.
{{cite web}}:CS1 maint: archived copy as title (link) - ^abcdefghConsumer Reports;Drug Effectiveness Review Project(March 2013),"Evaluating statin drugs to treat High Cholesterol and Heart Disease: Comparing Effectiveness, Safety, and Price"(PDF),Best Buy Drugs,Consumer Reports, p. 9,retrieved27 March2013,which cites
- United States Department of Health and Human Services;National Heart, Lung, and Blood Institute;National Institutes of Health(June 2005)."NHLBI, High Blood Cholesterol: What You Need to Know".nhlbi.nih.gov.Archived fromthe originalon 2013-04-01.Retrieved27 March2013.
{{cite web}}:CS1 maint: multiple names: authors list (link)
- United States Department of Health and Human Services;National Heart, Lung, and Blood Institute;National Institutes of Health(June 2005)."NHLBI, High Blood Cholesterol: What You Need to Know".nhlbi.nih.gov.Archived fromthe originalon 2013-04-01.Retrieved27 March2013.
- ^Research, Center for Drug Evaluation and."Drug Safety Information for Healthcare Professionals - Follow-up to the January 25, 2008 Early Communication about an Ongoing Data Review for Ezetimibe/Simvastatin (marketed as Vytorin), Ezetimibe (marketed as Zetia), and Simvastatin (marketed as Zocor)".Food and Drug Administration.
- ^Meyers CD, Kamanna VS, Kashyap ML (December 2004). "Niacin therapy in atherosclerosis".Current Opinion in Lipidology.15(6): 659–65.doi:10.1097/00041433-200412000-00006.PMID15529025.
- ^Soudijn W, van Wijngaarden I, Ijzerman AP (May 2007). "Nicotinic acid receptor subtypes and their ligands".Medicinal Research Reviews.27(3): 417–33.doi:10.1002/med.20102.PMID17238156.S2CID20876888.
- ^"WHO cooperative trial on primary prevention of ischemic heart disease with clofibrate to lower serum cholesterol: final mortality follow-up. Report of the Committee of Principal Investigators".Lancet.2(8403): 600–4. September 1984.doi:10.1016/s0140-6736(84)90595-6.PMID6147641.S2CID2473318.
- ^"TRILIPIX (fenofibric acid)"(PDF).Retrieved7 July2024.
- ^Song, B.L.; DeBose-Boyd, R.A. (2006)."Insig-Dependent Ubiquitination and Degradation of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Stimulated by Delta- and Gamma-Tocotrienols".J. Biol. Chem.281(35): 25054–25601.doi:10.1074/jbc.M605575200.PMID16831864.
- ^European Food Safety Authority, Journal (2010)."Scientific opinion on the substantiation of health claims related to plant sterols and plant stanols and maintenance of normal blood cholesterol concentrations".
- ^Trautwein, Elke; Vermeer, Mario; Hiemstra, Harry; Ras, Rouyanne (7 September 2018)."LDL-Cholesterol Lowering of Plant Sterols and Stanols—Which Factors Influence Their Efficacy?".Nutrients.10(9). MDPI AG: 1262.doi:10.3390/nu10091262.ISSN2072-6643.PMC6163911.PMID30205492.
- ^Cabral, Carlos Eduardo; Klein, Márcia Regina Simas Torres (2017)."Phytosterols in the Treatment of Hypercholesterolemia and Prevention of Cardiovascular Diseases".Arquivos Brasileiros de Cardiologia.109(5). Sociedade Brasileira de Cardiologia: 475–482.doi:10.5935/abc.20170158.ISSN0066-782X.PMC5729784.PMID29267628.
- ^Demonty, I.; Ras, R.T.; van der Knaap, H.C.; Duchateau, G.S.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. (Feb 2009)."Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake".The Journal of Nutrition.139(2): 271–84.doi:10.3945/jn.108.095125.PMID19091798.
- ^ab"Cholesterol Diet: How Nutrition & Foods Impact Levels".Cleveland Clinic.Retrieved2024-02-16.
- ^"Saturated Fat".www.heart.org.Retrieved2024-02-16.
- ^Nutrition, Center for Food Safety and Applied (2023-08-30)."Trans Fat".FDA.
- ^"Trans Fatty Acid - an overview | ScienceDirect Topics".www.sciencedirect.com.Retrieved2024-02-16.
- ^"Scientists Gene-Hacked Monkeys to Fix Their Cholesterol".Futurism.Retrieved13 June2021.
- ^Musunuru, Kiran; et al. (May 2021)."In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates".Nature.593(7859): 429–434.Bibcode:2021Natur.593..429M.doi:10.1038/s41586-021-03534-y.ISSN1476-4687.PMID34012082.S2CID234790939.Retrieved13 June2021.
External links
[edit]- Fat (LDL) Degradation:PMAPThe Proteolysis Map-animation
- Adult Treatment Panel III Full Report
- ATP III Update 2004
- O'Keefe JH, Cordain L, Harris WH, Moe RM, Vogel R (June 2004). "Optimal low-density lipoprotein is 50 to 70 mg/dL: lower is better and physiologically normal".Journal of the American College of Cardiology.43(11): 2142–6.doi:10.1016/j.jacc.2004.03.046.PMID15172426.