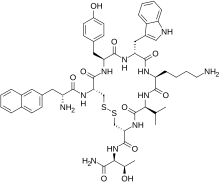
Lanreotide
 | |
| Clinical data | |
|---|---|
| Trade names | Somatuline |
| Other names | Lanreotide acetate (JANJP), Lanreotide acetate (USANUS) |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intramuscular,subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokineticdata | |
| Bioavailability | Approximately 80% |
| Protein binding | 78% |
| Metabolism | InGI tract |
| Eliminationhalf-life | 2 hours (immediate release) 5 days (sustained release) |
| Excretion | Mostly bile duct |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.215.992 |
| Chemical and physical data | |
| Formula | C54H69N11O10S2 |
| Molar mass | 1096.33g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lanreotide,sold under the brand nameSomatulineamong others, is amedicationused in the management ofacromegalyand symptoms caused byneuroendocrine tumors,most notablycarcinoid syndrome.It is a long-actinganalogueofsomatostatin,likeoctreotide.
Lanreotide (as lanreotideacetate) is manufactured byIpsen.It is available in several countries, including the United Kingdom, Australia and Canada, and was approved for sale in the United States by theFood and Drug Administration(FDA) on August 30, 2007.[2]
Medical uses
[edit]Lanreotide is used in the treatment ofacromegaly,due to bothpituitaryand non-pituitary growth hormone-secreting tumors, and the management of symptoms caused byneuroendocrine tumors,particularlycarcinoid tumorsandVIPomas.In the United States and Canada, lanreotide is only indicated for the treatment of acromegaly. In the United Kingdom, it is also indicated in the treatment ofthyrotrophicadenoma,[3]a rare tumor of the pituitary gland which secretes TSH.
Lanreotide also shows activity against non-endocrine tumors, and, along with othersomatostatin analogues,is being studied as a possible general antitumor agent.[4][5]
In December 2014, the US FDA approved lanreotide for the treatment of people with unresectable, well or moderately differentiated, locally advanced or metastatic gastroenteropancreaticneuroendocrine tumors(GEP-NETs).[6]
It is used for polycystic liver disease.[medical citation needed]It has also been shown that it reduces the volume by 264mls on average.[medical citation needed]
Side effects
[edit]The mainside effectsof lanreotide treatment are mild to moderate pain at the injection site andgastrointestinaldisturbances, such asdiarrhea,nauseaandvomiting.Isolated cases ofgallstone formationhave been associated with use of lanreotide, particularly over long periods of time.[3]
Pharmacology
[edit]Lanreotide is a synthetic analogue ofsomatostatin,a naturally occurring inhibitoryhormonewhich blocks the release of several other hormones, includinggrowth hormone,thyroid-stimulating hormone(TSH),insulinandglucagon.Lanreotide binds to the samereceptorsas somatostatin, although with higher affinity to peripheral receptors, and has similar activity. However, while somatostatin is quickly broken down in the body (within minutes),[7]lanreotide has a much longer half-life, and produces far more prolonged effects.[medical citation needed]
Formulations
[edit]Lanreotide is available in two formulations: a sustained release formulation (sold under the trade name 'Somatuline LA'), which isinjected intramuscularlyevery ten or fourteen days,[3]and an extended release formulation (UK trade name 'Somatuline Autogel', or 'SomatulineDepot' in the US), which is administered subcutaneously once a month.[8]
Self-assembling properties
[edit]Lanreotide has been shown to spontaneously self-assemble into monodisperse nanotubes of 24.4 nm diameter[9]and has been thereafter used as a fruitful and versatile model system in several biophysical studies.[citation needed]
References
[edit]- ^"Mytolac (Amdipharm Mercury Australia Pty Ltd)".Therapeutic Goods Administration (TGA).28 September 2022.Archivedfrom the original on 13 November 2022.Retrieved29 April2023.
- ^"FDA Approves New Drug to Treat Rare Disease, Acromegaly"(Press release). U.S.Food and Drug Administration.30 August 2007. Archived fromthe originalon 10 April 2021.Retrieved6 September2007.
- ^abc"Somatuline LA".electronic Medicines Compendium. 17 September 2003. Archived fromthe originalon 24 September 2006.Retrieved2 March2007.
- ^Kvols L, Woltering E (2006). "Role of somatostatin analogs in the clinical management of non-neuroendocrine solid tumors".Anticancer Drugs.17(6): 601–8.doi:10.1097/01.cad.0000210335.95828.ed.PMID16917205.
- ^Susini C, Buscail L (2006)."Rationale for the use of somatostatin analogs as antitumor agents".Ann Oncol.17(12): 1733–42.doi:10.1093/annonc/mdl105.PMID16801334.
- ^"FDA Approves Lanreotide Injection for GEP-NETs".2014. Archived fromthe originalon 26 June 2019.Retrieved29 April2023.
- ^Rens-Domiano S, Reisine T (1992). "Biochemical and functional properties of somatostatin receptors".J Neurochem.58(6): 1987–96.doi:10.1111/j.1471-4159.1992.tb10938.x.PMID1315373.S2CID36873846.
- ^"Somatuline Autogel".electronic Medicines Compendium. 12 April 2007. Archived fromthe originalon 28 September 2007.Retrieved19 April2007.
- ^Valéry C, Paternostre M, Robert B, Gulik-Krzywicki T, Narayanan T, Dedieu JC, Keller G, Torres ML, Cherif-Cheikh R, Calvo P, Artzner F (2003)."Biomimetic organization: Octapeptide self-assembly into nanotubes of viral capsid-like dimension".Proceedings of the National Academy of Sciences of the United States of America.100(18): 10258–62.Bibcode:2003PNAS..10010258V.doi:10.1073/pnas.1730609100.PMC193548.PMID12930900.
