Leukemia
| Leukemia | |
|---|---|
| Other names | Leukaemia |
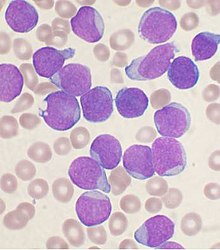 | |
| AWright's stainedbone marrow aspiratesmearfrom a person withB-cell acute lymphoblastic leukemia | |
| Pronunciation | |
| Specialty | Hematologyandoncology |
| Symptoms | Bleeding,bruising,fatigue,fever, increased risk of infections[2] |
| Usual onset | All ages,[3]most common in 60s and 70s.[4]It is the most common malignant cancer in children, but the cure rates are also higher for them. |
| Causes | Inheritedand environmental factors[5] |
| Risk factors | Smoking,family history,ionizing radiation,some chemicals such astrichloroethylene,prior chemotherapy,Down syndrome.[3][5] |
| Diagnostic method | Blood tests,bone marrow biopsy[2] |
| Treatment | Chemotherapy,radiation therapy,targeted therapy,bone marrow transplant,supportive care[3][6] |
| Prognosis | Five-year survival rate57% (U.S.)[4] |
| Frequency | 2.3 million (2015)[7] |
| Deaths | 353,500 (2015)[8] |
Leukemia(also spelledleukaemia;pronounced/luːˈkiːmiːə/[1]loo-KEE-mee-ə) is a group ofblood cancersthat usually begin in thebone marrowand produce high numbers of abnormalblood cells.[9]These blood cells are not fully developed and are calledblastsorleukemia cells.[2]Symptoms may include bleeding andbruising,bone pain,fatigue,fever,and an increased risk of infections.[2]These symptoms occur due to a lack of normalblood cells.[2]Diagnosis is typically made byblood testsorbone marrow biopsy.[2]
The exact cause of leukemia is unknown.[5]A combination ofgenetic factorsand environmental (non-inherited) factors are believed to play a role.[5]Risk factors includesmoking,ionizing radiation,petrochemicals (such asbenzene), prior chemotherapy, andDown syndrome.[5][3]People with a family history of leukemia are also at higher risk.[3]There are four main types of leukemia—acute lymphoblastic leukemia(ALL),acute myeloid leukemia(AML),chronic lymphocytic leukemia(CLL) andchronic myeloid leukemia(CML)—and a number of less common types.[3][10]Leukemias andlymphomasboth belong to a broader group oftumorsthat affect the blood, bone marrow, andlymphoid system,known astumors of the hematopoietic and lymphoid tissues.[11][12]
Treatment may involve some combination ofchemotherapy,radiation therapy,targeted therapy,andbone marrow transplant,withsupportiveandpalliative careprovided as needed.[3][6]Certain types of leukemia may be managed withwatchful waiting.[3]The success of treatment depends on the type of leukemia and the age of the patient. Outcomes have improved in the developed world.[10]Five-year survival ratewas 67% in the United States in the period from 2014 to 2020.[4]In children under 15 infirst-worldcountries, the five-year survival rate is greater than 60% or even 90%, depending on the type of leukemia.[13]In children who are cancer-free five years after diagnosis of acute leukemia, thecancer is unlikely to return.[13]
In 2015, leukemia was present in 2.3 million people worldwide and caused 353,500 deaths.[7][8]In 2012, it had newly developed in 352,000 people.[10]It is the most common type of cancer in children, with three-quarters of leukemia cases in children being the acute lymphoblastic type.[3]However, over 90% of all leukemias are diagnosed in adults, CLL and AML being most common.[3][14]It occurs more commonly in thedeveloped world.[10]
Classification
[edit]| Cell type | Acute | Chronic |
|---|---|---|
| Lymphocytic leukemia (or "lymphoblastic" ) |
Acute lymphoblastic leukemia (ALL) |
Chronic lymphocytic leukemia (CLL) |
| Myelogenous leukemia ( "myeloid" or "nonlymphocytic" ) |
Acute myelogenous leukemia (AML or myeloblastic) |
Chronic myelogenous leukemia (CML) |
General classification
[edit]Clinically and pathologically, leukemia is subdivided into a variety of large groups. The first division is between itsacuteandchronicforms:[15]
- Acute leukemiais characterized by a rapid increase in the number of immature blood cells. The crowding that results from such cells makes the bone marrow unable to produce healthy blood cells resulting in lowhemoglobinand lowplatelets.Immediate treatment is required in acute leukemia because of the rapid progression and accumulation of themalignant cells,which then spill over into the bloodstream and spread to other organs of the body. Acute forms of leukemia are the most common forms ofleukemia in children.
- Chronic leukemiais characterized by the excessive buildup of relatively mature, but still abnormal,white blood cells(or, more rarely,red blood cells). Typically taking months or years to progress, the cells are produced at a much higher rate than normal, resulting in many abnormal white blood cells. Whereas acute leukemia must be treated immediately, chronic forms are sometimes monitored for some time before treatment to ensure maximum effectiveness of therapy. Chronic leukemia mostly occurs in older people but can occur in any age group.
Additionally, the diseases are subdivided according to which kind of blood cell is affected. This divides leukemias intolymphoblasticorlymphocytic leukemiasandmyeloidormyelogenous leukemias:[15]
- In lymphoblastic orlymphocytic leukemias,the cancerous change takes place in a type of marrow cell that normally goes on to formlymphocytes,which are infection-fighting immune system cells. Most lymphocytic leukemias involve a specific subtype of lymphocyte, theB cell.
- In myeloid ormyelogenous leukemias,the cancerous change takes place in atype of marrow cellthat normally goes on to formred blood cells,some other types of white cells, andplatelets.
Combining these two classifications provides a total of four main categories. Within each of these main categories, there are typically several subcategories. Finally, some rarer types are usually considered to be outside of this classification scheme.[15][16]
Specific types
[edit]- Acute lymphoblastic leukemia(ALL) is the most common type of leukemia in young children. It also affects adults, especially those 65 and older. Standard treatments involvechemotherapyandradiotherapy.Subtypes includeprecursor B acute lymphoblastic leukemia,precursor T acute lymphoblastic leukemia,Burkitt's leukemia,andacute biphenotypic leukemia.While most cases of ALL occur in children, 80% of deaths from ALL occur in adults.[17]
- Chronic lymphocytic leukemia(CLL) most often affects adults over the age of 55. It sometimes occurs in younger adults, but it almost never affects children. Two-thirds of affected people are men. The five-year survival rate is 85%.[18]It is incurable, but there are many effective treatments. One subtype isB-cell prolymphocytic leukemia,a more aggressive disease.
- Acute myelogenous leukemia(AML) occurs far more commonly in adults than in children, and more commonly in men than women. It is treated with chemotherapy. The five-year survival rate is 20%.[19]Subtypes of AML includeacute promyelocytic leukemia,acute myeloblastic leukemia,andacute megakaryoblastic leukemia.
- Chronic myelogenous leukemia(CML) occurs mainly in adults; a very small number of children also develop this disease. It is treated withimatinib(Gleevec in United States, Glivec in Europe) or other drugs.[20]The five-year survival rate is 90%.[21][22]One subtype ischronic myelomonocytic leukemia.
- Hairy cell leukemia(HCL) is sometimes considered a subset of chronic lymphocytic leukemia, but does not fit neatly into this category. About 80% of affected people are adult men. No cases in children have been reported. HCL is incurable but easily treatable. Survival is 96% to 100% at ten years.[23]
- T-cell prolymphocytic leukemia(T-PLL) is a very rare and aggressive leukemia affecting adults; somewhat more men than women are diagnosed with this disease.[24]Despite its overall rarity, it is the most common type of matureT cellleukemia;[25]nearly all other leukemias involveB cells.It is difficult to treat, and the median survival is measured in months.
- Large granular lymphocytic leukemiamay involve either T-cells orNK cells;like hairy cell leukemia, which involves solely B cells, it is a rare andindolent(not aggressive) leukemia.[26]
- Adult T-cell leukemiais caused byhuman T-lymphotropic virus(HTLV), a virus similar toHIV.Like HIV, HTLV infects CD4+ T-cells and replicates within them; however, unlike HIV, it does not destroy them. Instead, HTLV "immortalizes" the infected T-cells, giving them the ability to proliferate abnormally. Human T-cell lymphotropic virus types I and II (HTLV-I/II) are endemic in certain areas of the world.[citation needed]
- Clonal eosinophilias(also calledclonal hypereosinophilias) are a group of blood disorders characterized by the growth ofeosinophilsin thebone marrow,blood, and/or other tissues. They may bepre-cancerousorcancerous.Clonal eosinophilias involve a"clone"of eosinophils, i.e., a group of genetically identical eosinophils that all grew from the samemutatedancestor cell.[27]These disorders may evolve intochronic eosinophilic leukemiaor may be associated with various forms ofmyeloidneoplasms,lymphoidneoplasms,myelofibrosis,or themyelodysplastic syndrome.[28][29][27]
Pre-leukemia
[edit]- Transient myeloproliferative disease,also termed transient leukemia, involves the abnormal proliferation of acloneof non-cancerousmegakaryoblasts.The disease is restricted to individuals withDown syndromeor genetic changes similar to those in Down syndrome, develops in a baby during pregnancy or shortly after birth, and resolves within 3 months or, in ~10% of cases, progresses toacute megakaryoblastic leukemia.Transient myeloid leukemia is a pre-leukemic condition.[30][31][32]
Signs and symptoms
[edit]
The most common symptoms in children are easybruising,pale skin,fever,and anenlarged spleenorliver.[34]
Damage to the bone marrow, by way of displacing the normal bone marrow cells with higher numbers of immature white blood cells, results in a lack of bloodplatelets,which are important in theblood clottingprocess. This means people with leukemia may easily becomebruised,bleedexcessively, or develop pinprick bleeds (petechiae).[35]
White blood cells,which are involved in fightingpathogens,may be suppressed or dysfunctional. This could cause the person's immune system to be unable to fight off a simple infection or to start attacking other body cells. Because leukemia prevents the immune system from working normally, some people experience frequentinfection,ranging from infectedtonsils,sores in the mouth,ordiarrheato life-threateningpneumoniaoropportunistic infections.[36]
Finally, the red blood cell deficiency leads toanemia,which may causedyspneaandpallor.[37]
Some people experience other symptoms, such as fevers, chills, night sweats, weakness in the limbs, feelingfatiguedand other commonflu-like symptoms.Some people experience nausea or a feeling of fullness due to an enlargedliverandspleen;this can result in unintentionalweight loss.Blastsaffected by the disease may come together and become swollen in the liver or in thelymph nodescausing pain and leading to nausea.[38]
If the leukemic cells invade thecentral nervous system,then neurological symptoms (notablyheadaches) can occur. Uncommon neurological symptoms likemigraines,seizures,orcomacan occur as a result of brain stem pressure. All symptoms associated with leukemia can be attributed to other diseases. Consequently, leukemia is always diagnosed throughmedical tests.
The wordleukemia,which means 'white blood', is derived from the characteristic high white blood cell count that presents in most affected people before treatment. The high number of white blood cells is apparent when a blood sample isviewed under a microscope,with the extra white blood cells frequently being immature or dysfunctional. The excessive number of cells can also interfere with the level of other cells, causing further harmful imbalance in the blood count.[39]
Some people diagnosed with leukemia do not have high white blood cell counts visible during a regular blood count. This less-common condition is calledaleukemia.The bone marrow still contains cancerous white blood cells that disrupt the normal production of blood cells, but they remain in the marrow instead of entering the bloodstream, where they would be visible in a blood test. For a person with aleukemia, the white blood cell counts in the bloodstream can be normal or low. Aleukemia can occur in any of the four major types of leukemia, and is particularly common inhairy cell leukemia.[40]
Causes
[edit]Studies in 2009 and 2010 have shown a positive correlation between exposure toformaldehydeand the development of leukemia, particularlymyeloid leukemia.[41][42]The different leukemias likely have different causes.[43]
Leukemia, like other cancers, results frommutationsin theDNA.Certain mutations can trigger leukemia by activatingoncogenesor deactivatingtumor suppressor genes,and thereby disrupting the regulation of cell death, differentiation or division. These mutations may occur spontaneously or as a result of exposure toradiationorcarcinogenicsubstances.[44]
Among adults, the known causes are natural and artificialionizing radiationand petrochemicals, notablybenzeneand alkylatingchemotherapyagents for previous malignancies.[45][46][47]Use oftobaccois associated with a small increase in the risk of developingacute myeloid leukemiain adults.[45]Cohort and case-control studies have linked exposure to somepetrochemicalsandhair dyesto the development of some forms of leukemia. Diet has very limited or no effect, although eating more vegetables may confer a small protective benefit.[48]
Viruses have also been linked to some forms of leukemia. For example,human T-lymphotropic virus(HTLV-1) causesadult T-cell leukemia.[49]
A few cases ofmaternal-fetal transmission(a baby acquires leukemia because its mother had leukemia during the pregnancy) have been reported.[45]Children born to mothers who usefertility drugsto induce ovulation are more than twice as likely to develop leukemia during their childhoods than other children.[50]
In a recent systematic review and meta-analysis of any type of leukemia in neonates usingphototherapy,typically to treatneonatal jaundice,a statistically significant association was detected between using phototherapy and myeloid leukemia. However, it is still questionable whether phototherapy is genuinely the cause of cancer or simply a result of the same underlying factors that gave rise to cancer.[51]
Radiation
[edit]Large doses ofSr-90(called abone seekingradioisotope) fromnuclear reactoraccidents, increases the risk ofbone cancerand leukemia in animals and is presumed to do so in people.[52]
Genetic conditions
[edit]Some people have a genetic predisposition towards developing leukemia. This predisposition is demonstrated by family histories andtwin studies.[45]The affected people may have a single gene or multiple genes in common. In some cases, families tend to develop the same kinds of leukemia as other members; in other families, affected people may develop different forms ofleukemia or related blood cancers.[45]
In addition to these genetic issues, people with chromosomal abnormalities or certain other genetic conditions have a greater risk of leukemia.[46]For example, people withDown syndromehave a significantly increased risk of developing forms of acute leukemia (especiallyacute myeloid leukemia), andFanconi anemiais a risk factor for developing acute myeloid leukemia.[45]Mutation inSPRED1 genehas been associated with a predisposition to childhood leukemia.[53]
Chronic myelogenous leukemiais associated with a genetic abnormality called thePhiladelphia translocation;95% of people with CML carry the Philadelphia mutation, although this is not exclusive to CML and can be observed in people with other types of leukemia.[54][55][56][57]
Non-ionizing radiation
[edit]Whether or not non-ionizing radiation causes leukemia has been studied for several decades. TheInternational Agency for Research on Cancerexpert working group undertook a detailed review of all data on static andextremely low frequencyelectromagnetic energy, which occurs naturally and in association with the generation, transmission, and use of electrical power.[58]They concluded that there is limited evidence that high levels ofELFmagnetic (but not electric) fields might cause some cases ofchildhood leukemia.[58]No evidence for a relationship to leukemia or another form of malignancy in adults has been demonstrated.[58]Since exposure to such levels of ELFs is relatively uncommon, theWorld Health Organizationconcludes that ELF exposure, if later proven to be causative, would account for just 100 to 2400 cases worldwide each year, representing 0.2 to 4.9% of the total incidence of childhood leukemia for that year (about 0.03 to 0.9% of all leukemias).[59]
Diagnosis
[edit]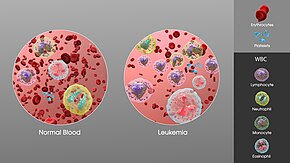
Diagnosis is usually based on repeatedcomplete blood countsand abone marrow examinationfollowing observations of the symptoms. Sometimes, blood tests may not show that a person has leukemia, especially in the early stages of the disease or during remission. Alymph node biopsycan be performed to diagnose certain types of leukemia in certain situations.[60]
Following diagnosis, blood chemistry tests can be used to determine the degree of liver and kidney damage or the effects of chemotherapy on the person. When concerns arise about other damages due to leukemia, doctors may use anX-ray,MRI,orultrasound.These can potentially show leukemia's effects on such body parts as bones (X-ray), the brain (MRI), or the kidneys, spleen, and liver (ultrasound).CT scanscan be used to check lymph nodes in the chest, though this is uncommon.[61]
Despite the use of these methods to diagnose whether or not a person has leukemia, many people have not been diagnosed because many of the symptoms are vague,non-specific,and can refer to other diseases. For this reason, the American Cancer Society estimates that at least one-fifth of the people with leukemia have not yet been diagnosed.[40]
Treatment
[edit]Most forms of leukemia are treated with pharmaceuticalmedication,typically combined into a multi-drugchemotherapy regimen.Some are also treated withradiation therapy.In some cases, abone marrow transplantis effective.
Acute lymphoblastic
[edit]Management of ALL is directed towards control of bone marrow and systemic (whole-body) disease. Additionally, treatment must prevent leukemic cells from spreading to other sites, particularly thecentral nervous system(CNS) e.g. monthly lumbar punctures.[clarification needed]In general, ALL treatment is divided into several phases:
- Induction chemotherapyto bring about bone marrow remission. For adults, standard induction plans includeprednisone,vincristine,and ananthracyclinedrug; other drug plans may includeL-asparaginaseorcyclophosphamide.For children with low-risk ALL, standard therapy usually consists of three drugs (prednisone, L-asparaginase, and vincristine) for the first month of treatment.
- Consolidation therapyorintensification therapyto eliminate any remaining leukemia cells. There are many different approaches to consolidation, but it is typically a high-dose, multi-drug treatment that is undertaken for a few months. People with low- to average-risk ALL receive therapy withantimetabolitedrugs such asmethotrexateand6-mercaptopurine(6-MP). People who are high-risk receive higher drug doses of these drugs, plus additional drugs.
- CNS prophylaxis(preventive therapy) to stop cancer from spreading to the brain and nervous system in high-risk people. Standardprophylaxismay include radiation of the head and/or drugs delivered directly into the spine.
- Maintenance treatmentswith chemotherapeutic drugs to prevent disease recurrence once remission has been achieved. Maintenance therapy usually involves lower drug doses and may continue for up to three years.
- Alternatively,allogeneic bone marrow transplantationmay be appropriate for high-risk or relapsed people.[62]
Chronic lymphocytic
[edit]Decision to treat
[edit]Hematologistsbase CLL treatment on both the stage and symptoms of the individual person. A large group of people with CLL have low-grade disease, which does not benefit from treatment. Individuals with CLL-related complications or more advanced disease often benefit from treatment. In general, the indications for treatment are:
- Fallinghemoglobinorplateletcount
- Progression to a later stage of disease
- Painful, disease-related overgrowth oflymph nodesorspleen
- An increase in the rate oflymphocyteproduction[63]
Treatment approach
[edit]Most CLL cases are incurable by present treatments, so treatment is directed towards suppressing the disease for many years, rather than curing it. The primary chemotherapeutic plan iscombinationchemotherapy withchlorambucilorcyclophosphamide,plus acorticosteroidsuch asprednisoneorprednisolone.The use of a corticosteroid has the additional benefit of suppressing some related autoimmune diseases, such asimmunohemolytic anemiaorimmune-mediated thrombocytopenia.In resistant cases,single-agenttreatments with nucleoside drugs such asfludarabine,[64]pentostatin,orcladribinemay be successful. Younger and healthier people may chooseallogeneicorautologousbone marrow transplantationin the hope of a permanent cure.[65]
Acute myelogenous
[edit]Many different anti-cancer drugs are effective for the treatment of AML. Treatments vary somewhat according to the age of the person and according to the specific subtype of AML. Overall, the strategy is to control bone marrow and systemic (whole-body) disease, while offering specific treatment for the central nervous system (CNS), if involved.[66]
In general, most oncologists rely on combinations of drugs for the initial,induction phaseof chemotherapy. Such combination chemotherapy usually offers the benefits of earlyremissionand a lower risk of disease resistance.Consolidationandmaintenancetreatments are intended to prevent disease recurrence. Consolidation treatment often entails a repetition of induction chemotherapy or the intensification of chemotherapy with additional drugs. By contrast, maintenance treatment involves drug doses that are lower than those administered during the induction phase.[67]
Chronic myelogenous
[edit]There are many possible treatments for CML, but the standard of care for newly diagnosed people isimatinib(Gleevec) therapy.[68]Compared to most anti-cancer drugs, it has relatively few side effects and can be takenorallyat home. With this drug, more than 90% of people will be able to keep the disease in check for at least five years,[68]so that CML becomes a chronic, manageable condition.
In a more advanced, uncontrolled state, when the person cannot tolerate imatinib, or if the person wishes to attempt a permanent cure, then an allogeneic bone marrow transplantation may be performed. This procedure involves high-dose chemotherapy and radiation followed by infusion of bone marrow from a compatible donor. Approximately 30% of people die from this procedure.[68]
Hairy cell
[edit]Decision to treat
People with hairy cell leukemia who are symptom-free typically do not receive immediate treatment. Treatment is generally considered necessary when the person shows signs and symptoms such as low blood cell counts (e.g., infection-fighting neutrophil count below 1.0 K/μL), frequent infections, unexplained bruises, anemia, or fatigue that is significant enough to disrupt the person's everyday life.[69]
Typical treatment approach
People who need treatment usually receive either one week ofcladribine,given daily by intravenous infusion or a simple injection under the skin, or six months ofpentostatin,given every four weeks by intravenous infusion. In most cases, one round of treatment will produce a prolonged remission.[70]
Other treatments includerituximabinfusion or self-injection withInterferon-alpha.In limited cases, the person may benefit fromsplenectomy(removal of thespleen). These treatments are not typically given as the first treatment because their success rates are lower than cladribine or pentostatin.[71]
T-cell prolymphocytic
[edit]Most people with T-cell prolymphocytic leukemia, a rare and aggressive leukemia with a median survival of less than one year, require immediate treatment.[72]
T-cell prolymphocytic leukemia is difficult to treat, and it does not respond to most available chemotherapeutic drugs.[72]Many different treatments have been attempted, with limited success in certain people:purine analogues(pentostatin, fludarabine, cladribine),chlorambucil,and various forms of combination chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisoneCHOP,cyclophosphamide, vincristine, prednisone [COP], vincristine, doxorubicin, prednisone, etoposide, cyclophosphamide, bleomycinVAPEC-B).Alemtuzumab(Campath), amonoclonal antibodythat attacks white blood cells, has been used in treatment with greater success than previous options.[72]
Some people who successfully respond to treatment also undergostem cell transplantationto consolidate the response.[72]
Juvenile myelomonocytic
[edit]Treatment forjuvenile myelomonocytic leukemiacan includesplenectomy,chemotherapy,andbone marrow transplantation.[73]
Prognosis
[edit]The success of treatment depends on the type of leukemia and the age of the person. Outcomes have improved in the developed world.[10]The averagefive-year survival rateis 65% in the United States.[4]In children under 15, the five-year survival rate is greater (60 to 85%), depending on the type of leukemia.[13]In children with acute leukemia who are cancer-free after five years, the cancer is unlikely to return.[13]
Outcomes depend on whether it is acute or chronic, the specific abnormal white blood cell type, the presence and severity ofanemiaorthrombocytopenia,the degree of tissue abnormality, the presence ofmetastasisandlymph nodeandbone marrowinfiltration, the availability of therapies and the skills of the health care team. Treatment outcomes may be better when people are treated at larger centers with greater experience.[74]
Epidemiology
[edit]
In 2010, globally, approximately 281,500 people died of leukemia.[75]In 2000, approximately 256,000 children and adults around the world developed a form of leukemia, and 209,000 died from it.[76]This represents about 3% of the almost seven million deaths due to cancer that year, and about 0.35% of all deaths from any cause.[76]Of the sixteen separate sites the body compared, leukemia was the 12th most common class ofneoplastic diseaseand the 11th most common cause of cancer-related death.[76]Leukemia occurs more commonly in thedeveloped world.[77]
United States
[edit]About 245,000 people in the United States are affected with some form of leukemia, including those that have achieved remission or cure. Rates from 1975 to 2011 have increased by 0.7% per year among children.[78]Approximately 44,270 new cases of leukemia were diagnosed in the year 2008 in the US.[79]This represents 2.9% of all cancers (excluding simple basal cell and squamous cell skin cancers) in the United States, and 30.4% of allblood cancers.[80]
Among children with some form of cancer, about a third have a type of leukemia, most commonlyacute lymphoblastic leukemia.[79]A type of leukemia is the second most common form of cancer in infants (under the age of 12 months) and the most common form of cancer in older children.[81]Boys are somewhat more likely to develop leukemia than girls, and white American children are almost twice as likely to develop leukemia than black American children.[81]Only about 3% cancer diagnoses among adults are for leukemias, but because cancer is much more common among adults, more than 90% of all leukemias are diagnosed in adults.[79]
Raceis arisk factorin the United States.Hispanics,especially those under the age of 20, are at the highest risk for leukemia, whilewhites,Native Americans,Asian Americans,andAlaska Nativesare at higher risk thanAfrican Americans.[82]
More men than women are diagnosed with leukemia and die from the disease. Around 30 percent more men than women have leukemia.[83]
Australia
[edit]In Australia, leukemia is the eleventh most common cancer.[84]In 2014–2018, Australians diagnosed with leukaemia had a 64% chance (65% for males and 64% for females) of surviving for five years compared to the rest of the Australian population–there was a 21% increase in survival rates between 1989–1993.[84]
UK
[edit]Overall, leukemia is the eleventh most common cancer in the UK (around 8,600 people were diagnosed with the disease in 2011), and it is the ninth most common cause of cancer death (around 4,800 people died in 2012).[85]
History
[edit]
Leukemia was first described by anatomist and surgeonAlfred-Armand-Louis-Marie Velpeauin 1827. A more complete description was given by pathologistRudolf Virchowin 1845. Around ten years after Virchow's findings, pathologistFranz Ernst Christian Neumannfound that the bone marrow of a deceased person with leukemia was colored "dirty green-yellow" as opposed to the normal red. This finding allowed Neumann to conclude that a bone marrow problem was responsible for the abnormal blood of people with leukemia.[86]
By 1900, leukemia was viewed as a family of diseases as opposed to a single disease. By 1947, Boston pathologistSidney Farberbelieved from past experiments thataminopterin,a folic acid mimic, could potentially cure leukemia in children. The majority of the children with ALL who were tested showed signs of improvement in their bone marrow, but none of them were actually cured. Nevertheless, this result did lead to further experiments.[87]
In 1962, researchers Emil J. Freireich, Jr. and Emil Frei III used combination chemotherapy to attempt to cure leukemia. The tests were successful with some people surviving long after the tests.[88]
Etymology
[edit]Observing an abnormally large number of white blood cells in a blood sample from a person, Virchow called the conditionLeukämieinGerman,which he formed from the twoGreekwordsleukos(λευκός), meaning 'white', andhaima(αἷμα), meaning 'blood'.[89]It was formerly also calledleucemia.[90]
Society and culture
[edit]According toSusan Sontag,leukemia was often romanticized in 20th-century fiction, portrayed as a joy-ending, clean disease whose fair, innocent and gentle victims die young or at the wrong time. As such, it was the cultural successor totuberculosis,which held this cultural position until it was discovered to be an infectious disease.[91]The 1970 romance novelLove Storyis an example of this romanticization of leukemia.[92]
In the United States, around $5.4 billion is spent on treatment a year.[93]
Research directions
[edit]Significant research into the causes, prevalence, diagnosis, treatment, and prognosis of leukemia is being performed. Hundreds ofclinical trialsare being planned or conducted at any given time.[94]Studies may focus on effective means of treatment, better ways of treating the disease, improving the quality of life for people, or appropriate care inremissionor after cures.[95]
In general, there are two types of leukemia research: clinical ortranslational researchandbasic research.Clinical/translational research focuses on studying the disease in a defined and generally immediately applicable way, such as testing a new drug in people. By contrast, basic science research studies the disease process at a distance, such as seeing whether a suspected carcinogen can cause leukemic changes in isolated cells in the laboratory or how the DNA changes inside leukemia cells as the disease progresses. The results from basic research studies are generally less immediately useful to people with the disease.[96]
Treatment throughgene therapyis currently being pursued. One such approach used genetically modifiedT cells,known aschimeric antigen receptor T cells(CAR-T cells), to attack cancer cells. In 2011, a year after treatment, two of the three people with advanced chronic lymphocytic leukemia were reported to be cancer-free[97]and in 2013, three of five subjects who had acute lymphocytic leukemia were reported to be in remission for five months to two years.[98]Subsequent studies with a variety of CAR-T types continue to be promising.[99]As of 2018, two CAR-T therapies have been approved by theFood and Drug Administration.CAR-T treatment has significant side effects,[100]and loss of theantigentargeted by the CAR-T cells is a common mechanism for relapse.[99]The stem cells that cause different types of leukemia are also being researched.[101]
Pregnancy
[edit]Leukemia is rarely associated with pregnancy, affecting only about 1 in 10,000 pregnant women.[102]How it is handled depends primarily on the type of leukemia. Nearly all leukemias appearing in pregnant women are acute leukemias.[103]Acute leukemias normally require prompt, aggressive treatment, despite significant risks ofpregnancy lossandbirth defects,especially if chemotherapy is given during the developmentally sensitivefirst trimester.[102]Chronic myelogenous leukemia can be treated with relative safety at any time during pregnancy withInterferon-alphahormones.[102]Treatment for chronic lymphocytic leukemias, which are rare in pregnant women, can often be postponed until after the end of the pregnancy.[102][103]
See also
[edit]- Acute erythroid leukemia
- Antileukemic drugs,medications used to kill leukemia cells
- Cancer-related fatigue
- Hematologic diseases,the large class of blood-related disorders, including leukemia
- Multiple myeloma
References
[edit]- ^ab"Leukemia".Merriam-Webster.30 May 2023.
- ^abcdef"What You lNeed To Know About Leukemia".National Cancer Institute.23 December 2013.Archivedfrom the original on 6 July 2014.Retrieved18 June2014.
- ^abcdefghij"A Snapshot of Leukemia".NCI.Archivedfrom the original on 4 July 2014.Retrieved18 June2014.
- ^abcd"SEER Stat Fact Sheets: Leukemia".National Cancer Institute. 2024.Updated as required.
- ^abcdeHutter JJ (June 2010). "Childhood leukemia".Pediatrics in Review.31(6): 234–241.doi:10.1542/pir.31-6-234.PMID20516235.S2CID207170780.
- ^abCordo' V, van der Zwet JC, Canté-Barrett K, Pieters R, Meijerink JP (January 2021)."T-cell Acute Lymphoblastic Leukemia: A Roadmap to Targeted Therapies".Blood Cancer Discovery.2(1): 19–31.doi:10.1158/2643-3230.BCD-20-0093.PMC8447273.PMID34661151.
- ^abVos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. (October 2016)."Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015".Lancet.388(10053): 1545–1602.doi:10.1016/S0140-6736(16)31678-6.PMC5055577.PMID27733282.
- ^abWang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. (October 2016)."Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015".Lancet.388(10053): 1459–1544.doi:10.1016/s0140-6736(16)31012-1.PMC5388903.PMID27733281.
- ^"Leukemia".NCI.1 January 1980.Archivedfrom the original on 27 May 2014.Retrieved13 June2014.
Cancer that starts in blood-forming tissue, such as the bone marrow, and causes large numbers of abnormal blood cells
- ^abcdeWorld Cancer Report 2014.World Health Organization. 2014. pp. Chapter 5.13.ISBN978-92-832-0429-9.
- ^Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. (July 2009)."The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes".Blood.114(5): 937–951.doi:10.1182/blood-2009-03-209262.PMID19357394.S2CID3101472.
- ^Baba AI, Câtoi C, eds. (2007).Comparative oncology.Bucharest: The Publishing House of the Romanian Academy. p. Chapter 17.ISBN978-973-27-1457-7.Archivedfrom the original on 10 September 2017.
- ^abcdAmerican Cancer Society (2 March 2014)."Survival rates for childhood leukemia".Archivedfrom the original on 14 July 2014.
- ^"Chronic Lymphocytic Leukemia (CLL) – Hematology and Oncology".MSD Manual Professional Edition.Retrieved1 February2020.
- ^abc"Questions and Answers About Leukemia"(PDF).Centers for Disease Control and Prevention.Archived(PDF)from the original on 30 July 2021.Retrieved8 August2021.
- ^"Leukemia – Symptoms and causes".Mayo Clinic.Retrieved8 August2021.
- ^"Key Statistics for Acute Lymphocytic Leukemia (ALL)".American Cancer Society.8 January 2019.Retrieved9 December2019.
- ^"Finding Cancer Statistics » Cancer Stat Fact Sheets »Chronic Lymphocytic Leukemia".National Cancer Institute.Archived fromthe originalon 16 April 2008.
- ^"Survival: Acute Myeloid Leukaemia".Cancer Research UK.10 July 2019.Retrieved2 December2019.
- ^"Novartis Oncology".Archivedfrom the original on 5 November 2013.
- ^Moyer P (12 June 2006)."Patients with Chronic Myelogenous Leukemia Continue to Do Well on Imatinib at 5-Year Follow-Up".Medscape Medical News.Archived fromthe originalon 15 May 2013.
- ^"Updated Results of Tyrosine Kinase Inhibitors in CML".ASCO 2006 Conference Summaries.Archived fromthe originalon 29 December 2007.
- ^Else M, Ruchlemer R, Osuji N, Del Giudice I, Matutes E, Woodman A, et al. (December 2005)."Long remissions in hairy cell leukemia with purine analogs: a report of 219 patients with a median follow-up of 12.5 years".Cancer.104(11): 2442–2448.doi:10.1002/cncr.21447.PMID16245328.S2CID43282431.
- ^Matutes E (January 1998)."T-cell Prolymphocytic Leukemia".Cancer Control.5(1): 19–24.doi:10.1177/107327489800500102.PMID10761013.Archivedfrom the original on 11 February 2009.
- ^Valbuena JR, Herling M, Admirand JH, Padula A, Jones D, Medeiros LJ (March 2005)."T-cell prolymphocytic leukemia involving extramedullary sites".American Journal of Clinical Pathology.123(3): 456–464.doi:10.1309/93P4-2RNG-5XBG-3KBE.PMID15716243.Archivedfrom the original on 15 May 2013.
- ^Jaffe ES, Harris NL, Stein H, Vardiman JW, et al. (World Health Organization, International Agency for Research on Cancer) (2001).Pathology and genetics of tumours of haematopoietic and lymphoid tissues.World Health Organization Classification of Tumors. Vol. 3. Lyon: IARC Press.ISBN978-92-832-2411-2.
- ^abReiter A, Gotlib J (February 2017)."Myeloid neoplasms with eosinophilia".Blood.129(6): 704–714.doi:10.1182/blood-2016-10-695973.PMID28028030.
- ^Gotlib J (November 2015)."World Health Organization-defined eosinophilic disorders: 2015 update on diagnosis, risk stratification, and management".American Journal of Hematology.90(11): 1077–1089.doi:10.1002/ajh.24196.PMID26486351.S2CID42668440.
- ^Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. (May 2016)."The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia".Blood.127(20): 2391–2405.doi:10.1182/blood-2016-03-643544.PMID27069254.S2CID18338178.
- ^Bhatnagar N, Nizery L, Tunstall O, Vyas P, Roberts I (October 2016)."Transient Abnormal Myelopoiesis and AML in Down Syndrome: an Update".Current Hematologic Malignancy Reports.11(5): 333–341.doi:10.1007/s11899-016-0338-x.PMC5031718.PMID27510823.
- ^Crispino JD, Horwitz MS (April 2017)."GATA factor mutations in hematologic disease".Blood.129(15): 2103–2110.doi:10.1182/blood-2016-09-687889.PMC5391620.PMID28179280.
- ^Seewald L, Taub JW, Maloney KW, McCabe ER (September 2012). "Acute leukemias in children with Down syndrome".Molecular Genetics and Metabolism.107(1–2): 25–30.doi:10.1016/j.ymgme.2012.07.011.PMID22867885.
- ^Reference list is found atimage description page in Wikimedia Commons
- ^Clarke RT, Van den Bruel A, Bankhead C, Mitchell CD, Phillips B, Thompson MJ (October 2016)."Clinical presentation of childhood leukaemia: a systematic review and meta-analysis".Archives of Disease in Childhood.101(10): 894–901.doi:10.1136/archdischild-2016-311251.PMID27647842.
- ^Jyothi KT, Subrahmanyam PS, Sravanthi AC (July 2017). "Application of Differential Equations in Medical Science".Research Journal of Science and Technology.9(3): 425–426.doi:10.5958/2349-2988.2017.00074.2.
- ^"Types of Leukemia: Common, Rare and More Varieties".Cancer Treatment Centers of America.5 October 2018.Retrieved8 October2021.
- ^"Iron deficiency anemia – Symptoms and causes".Mayo Clinic.Retrieved5 March2022.
- ^"Leukemia".Columbia Electronic Encyclopedia, 6th Edition.Retrieved4 November2011.
- ^"Leukemia: Symptoms, Signs, Causes, Types & Treatment".Cleveland Clinic.Retrieved13 October2022.
- ^abAmerican Cancer Society (2010)."How is Leukemia Diagnosed?".Detailed Guide: Leukemia – Adult Chronic.American Cancer Society. Archived fromthe originalon 5 April 2010.Retrieved4 May2010.
- ^Zhang L, Steinmaus C, Eastmond E, Xin X, Smith S (March–June 2009)."Formaldehyde exposure and leukemia: A new meta-analysis and potential mechanisms"(PDF).Mutation Research/Reviews in Mutation Research.681(2–3): 150–168.doi:10.1016/j.mrrev.2008.07.002.PMID18674636.Archived fromthe original(PDF)on 27 March 2014.Retrieved22 May2013.
- ^Zhang L, Freeman LE, Nakamura J, Hecht SS, Vandenberg JJ, Smith MT, et al. (2010)."Formaldehyde and Leukemia: Epidemiology, Potential Mechanisms, and Implications for Risk Assessment".Environmental and Molecular Mutagenesis.51(3): 181–191.Bibcode:2010EnvMM..51..181Z.doi:10.1002/em.20534.PMC2839060.PMID19790261.
- ^Novak EM, Rego EM, eds. (2012).Physiopathogenesis of Hematological Cancer.Bentham Science Publishers.ISBN978-1-60805-259-2.
- ^Radivoyevitch T, Sachs RK, Gale RP, Molenaar RJ, Brenner DJ, Hill BT, et al. (February 2016). "Defining AML and MDS second cancer risk dynamics after diagnoses of first cancers treated or not with radiation".Leukemia.30(2): 285–294.doi:10.1038/leu.2015.258.PMID26460209.S2CID22100511.
- ^abcdefWiernik PH (2001).Adult leukemias.New York: B. C. Decker. pp. 3–15.ISBN978-1-55009-111-3.
- ^abRobinette MS, Cotter S, Van de Water D (2001).Quick Look Series in Veterinary Medicine: Hematology.Teton NewMedia. p. 105.ISBN978-1-893441-36-1.
- ^Stass SA, Schumacher HR, Rock WR (2000).Handbook of hematologic pathology.New York, N.Y: Marcel Dekker. pp.193–194.ISBN978-0-8247-0170-3.
- ^Ross JA, Kasum CM, Davies SM, Jacobs DR, Folsom AR, Potter JD (August 2002)."Diet and risk of leukemia in the Iowa Women's Health Study".Cancer Epidemiology, Biomarkers & Prevention.11(8): 777–781.PMID12163333.Archivedfrom the original on 10 September 2017.
- ^Leonard B (1998).Leukemia: A Research Report.DIANE Publishing. p.7.ISBN978-0-7881-7189-5.
- ^Rudant J, Amigou A, Orsi L, Althaus T, Leverger G, Baruchel A, et al. (February 2013). "Fertility treatments, congenital malformations, fetal loss, and childhood acute leukemia: the ESCALE study (SFCE)".Pediatric Blood & Cancer.60(2): 301–308.doi:10.1002/pbc.24192.PMID22610722.S2CID26010916.
- ^Abdellatif, Mohammed, et al. "Association between neonatal phototherapy and future cancer: an updated systematic review and meta-analysis." European Journal of Pediatrics (2022): 1–13.
- ^"Backgrounder on Radiation Protection and the" Tooth Fairy "Issue".U.S. Nuclear Regulatory Commission. December 2004. Archived fromthe originalon 20 July 2017.
- ^Pasmant E, Ballerini P, Lapillonne H, Perot C, Vidaud D, Leverger G, et al. (July 2009)."SPRED1 disorder and predisposition to leukemia in children".Blood.114(5): 1131.doi:10.1182/blood-2009-04-218503.PMID19643996.
- ^Salesse S, Verfaillie CM (December 2002)."BCR/ABL: from molecular mechanisms of leukemia induction to treatment of chronic myelogenous leukemia".Oncogene.21(56): 8547–8559.doi:10.1038/sj.onc.1206082.PMID12476301.
- ^"NCI Dictionary of Cancer Terms".National Cancer Institute.2 February 2011.Archivedfrom the original on 16 February 2017.Retrieved15 February2017.
- ^"Do We Know What Causes Chronic Myeloid Leukemia?".www.cancer.org.Archivedfrom the original on 16 February 2017.Retrieved15 February2017.
- ^"What is chronic myeloid leukaemia? (CML) – Understanding – Macmillan Cancer Support".www.macmillan.org.uk.Archivedfrom the original on 16 February 2017.Retrieved15 February2017.
- ^abcNon-Ionizing Radiation, Part 1: Static and Extremely Low-Frequency (ELF) Electric and Magnetic Fields (IARC Monographs on the Evaluation of the Carcinogenic Risks).Geneva: World Health Organisation. 2002. pp. 332–333, 338.ISBN978-92-832-1280-5.Archivedfrom the original on 6 December 2008.
- ^"WHO | Electromagnetic fields and public health".Archived fromthe originalon 16 February 2009.Retrieved18 February2009.
- ^"Diagnosing Chronic Lymphocytic Leukemia in Adults".NYU Langone Health.Archivedfrom the original on 26 March 2021.Retrieved26 March2021.
- ^"Chronic Lymphocytic Leukemia (CLL): Tests After Diagnosis".University of Rochester Medical Center.Archivedfrom the original on 26 March 2021.Retrieved26 March2021.
- ^Hoffbrand AV, Moss PA, Pettit JE (2006).Essential haematology(5th ed.). Malden, Mass.: Blackwell Pub.ISBN978-1-4051-3649-5.
- ^National Cancer Institute (1 January 1980)."Chronic Lymphocytic Leukemia (PDQ) Treatment: Stage Information".Archivedfrom the original on 17 October 2007.Retrieved4 September2007.
- ^Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C, et al. (February 2006)."Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia".Blood.107(3): 885–891.doi:10.1182/blood-2005-06-2395.PMID16219797.
- ^Gribben JG (January 2009)."Stem cell transplantation in chronic lymphocytic leukemia".Biology of Blood and Marrow Transplantation.15(1 Suppl): 53–58.doi:10.1016/j.bbmt.2008.10.022.PMC2668540.PMID19147079.
- ^Baig MQ (22 March 2017).Practical Radiotherapy & Chemotherapy Planning.JP Medical Ltd.ISBN978-93-86150-01-1.
- ^American Cancer Society (22 March 2012)."Typical treatment of acute myeloid leukemia (except promyelocytic M3)".Detailed Guide: Leukemia – Acute Myeloid (AML).American Cancer Society.Archivedfrom the original on 12 November 2012.Retrieved31 October2012.
- ^abcFausel C (October 2007)."Targeted chronic myeloid leukemia therapy: seeking a cure"(PDF).Journal of Managed Care Pharmacy.13(8 Suppl A): 8–12.doi:10.18553/jmcp.2007.13.s8-a.8.PMC10437886.PMID17970609.Archived fromthe original(PDF)on 28 May 2008.Retrieved18 May2008.
- ^"Hairy Cell Leukemia".The Lecturio Medical Concept Library.Retrieved24 July2021.
- ^Robak T, Jamroziak K, Gora-Tybor J, Blonski JZ, Kasznicki M, Dwilewicz-Trojaczek J, et al. (May 2007)."Cladribine in a weekly versus daily schedule for untreated active hairy cell leukemia: final report from the Polish Adult Leukemia Group (PALG) of a prospective, randomized, multicenter trial".Blood.109(9): 3672–3675.doi:10.1182/blood-2006-08-042929.PMID17209059.
- ^Saven A, Burian C, Adusumalli J, Koziol JA (April 1999). "Filgrastim for cladribine-induced neutropenic fever in patients with hairy cell leukemia".Blood.93(8): 2471–2477.doi:10.1182/blood.V93.8.2471.PMID10194424.
- ^abcdDearden CE, Matutes E, Cazin B, Tjønnfjord GE, Parreira A, Nomdedeu B, et al. (September 2001)."High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H".Blood.98(6): 1721–1726.doi:10.1182/blood.V98.6.1721.PMID11535503.S2CID26664911.
- ^"JMMLfoundation.org".JMMLfoundation.org. Archived fromthe originalon 25 January 2009.Retrieved29 August2010.
- ^Stock W (2010)."Adolescents and young adults with acute lymphoblastic leukemia".Hematology. American Society of Hematology. Education Program.2010:21–29.doi:10.1182/asheducation-2010.1.21.PMID21239766.S2CID9796380.
- ^Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. (December 2012)."Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010".Lancet.380(9859): 2095–2128.doi:10.1016/S0140-6736(12)61728-0.hdl:10536/DRO/DU:30050819.PMC10790329.PMID23245604.S2CID1541253.
- ^abcMathers CD, Boschi-Pinto C, Lopez AD, Murray CJ (2001)."Cancer incidence, mortality and survival by site for 14 regions of the world"(PDF).Global Programme on Evidence for Health Policy Discussion Paper No. 13.World Health Organization.
- ^World Cancer Report 2014.World Health Organization. 2014. pp. Chapter 5.13.ISBN978-92-832-0429-9.
- ^Amitay EL, Keinan-Boker L (June 2015)."Breastfeeding and Childhood Leukemia Incidence: A Meta-analysis and Systematic Review".JAMA Pediatrics.169(6): e151025.doi:10.1001/jamapediatrics.2015.1025.PMID26030516.
- ^abc"Leukemia Facts & Statistics".The Leukemia & Lymphoma Society.Archived fromthe originalon 16 April 2009.Retrieved2 July2009.
- ^Horner MJ, Ries LA, Krapcho M, Neyman N, et al. (eds.)."SEER Cancer Statistics Review, 1975–2006".Surveillance Epidemiology and End Results (SEER).Bethesda, MD:National Cancer Institute.Archivedfrom the original on 26 September 2009.Retrieved3 November2009.
Table 1.4: Age-Adjusted SEER Incidence and U.S. Death Rates and 5-Year Relative Survival Rates By Primary Cancer Site, Sex and Time Period
- ^abGurney JG, Smith MA, Ross JA (1999)."Leukemia"(PDF).Cancer Incidence and Survival among Children and Adolescents, United States SEER program 1975–1995.Cancer Statistics Branch, National Cancer Institute. Archived fromthe original(PDF)on 24 December 2010.
- ^"Childhood Blood Cancers".The Leukemia & Lymphoma Society.Archived fromthe originalon 5 September 2012.
- ^"Facts 2012"(PDF).The Leukemia & Lymphoma Society.Archived fromthe originalon 14 October 2012.
- ^ab"Leukaemia in Australia statistics".canceraustralia.gov.au.2023.Retrieved3 April2023.
- ^"Leukaemia (all subtypes combined) statistics".Cancer Research UK.Archivedfrom the original on 7 October 2014.Retrieved27 October2014.
- ^Thomas X (6 August 2013)."First contributors in the history of leukemia".World Journal of Hematology.2(3): 62–70.doi:10.5315/wjh.v2.i3.62.
- ^Ribatti D (May 2012). "Sidney Farber and the treatment of childhood acute lymphoblastic leukemia with a chemotherapeutic agent".Pediatric Hematology and Oncology.29(4): 299–302.doi:10.3109/08880018.2012.678969.PMID22568792.S2CID31176962.
- ^Patlak M (March 2002)."Targeting leukemia: from bench to bedside".FASEB Journal.16(3): 273.doi:10.1096/fj.02-0029bkt.PMID11874976.S2CID221751708.
- ^"Leukemia: MedlinePlus Medical Encyclopedia".MedlinePlus.8 May 2019.Retrieved16 May2019.
- ^"leukemia".Online Etymology Dictionary.Retrieved14 February2023.
- ^Sontag S (1978).Illness as Metaphor.New York: Farrar, Straus and Giroux. pp.18.ISBN978-0-374-17443-9.
- ^Bey B (2017).Cancer as Metaphor: The Metaphorical Implications of Romanticized Illness in Young Adult Fiction(English Honors thesis).Trinity University.p. 5-6.
- ^"A Snapshot of Leukemia".NCI.Archivedfrom the original on 4 July 2014.Retrieved18 June2014.
- ^"Search of: leukemia — List Results — ClinicalTrials.gov".Archivedfrom the original on 15 September 2010.
- ^"Learn About Clinical Studies – Reasons for Conducting Clinical Studies".Clinicaltrials.gov.Retrieved3 March2023.
- ^"Understanding Clinical Trials for Blood Cancers"(PDF).Leukemia and Lymphoma Society. Archived fromthe original(PDF)on 5 January 2011.Retrieved19 May2010.
- ^Jaslow R (11 August 2011)."New Leukemia Therapy Destroys Cancer by Turning Blood Cells into" Assassins "".CBSnews.com HealthPop section.Archivedfrom the original on 31 March 2014.Retrieved11 August2011.
- ^Coghlan A (26 March 2013)."Gene therapy cures leukaemia in eight days".The New Scientist.Archived fromthe originalon 15 May 2015.Retrieved15 April2013.
- ^abZhao J, Song Y, Liu D (February 2019)."Clinical trials of dual-target CAR T cells, donor-derived CAR T cells, and universal CAR T cells for acute lymphoid leukemia".Journal of Hematology & Oncology.12(1): 17.doi:10.1186/s13045-019-0705-x.PMC6376657.PMID30764841.
- ^Zheng PP, Kros JM, Li J (June 2018)."Approved CAR T cell therapies: ice bucket challenges on glaring safety risks and long-term impacts".Drug Discovery Today.23(6): 1175–1182.doi:10.1016/j.drudis.2018.02.012.hdl:1765/105338.PMID29501911.
- ^"How we're beating leukaemia".Leukaemia & Lymphoma Research.Archivedfrom the original on 27 September 2013.Retrieved24 September2013.
- ^abcdShapira T, Pereg D, Lishner M (September 2008). "How I treat acute and chronic leukemia in pregnancy".Blood Reviews.22(5): 247–259.doi:10.1016/j.blre.2008.03.006.PMID18472198.
- ^abKoren G, Lishner M (2010)."Pregnancy and commonly used drugs in hematology practice".Hematology. American Society of Hematology. Education Program.2010:160–165.doi:10.1182/asheducation-2010.1.160.PMID21239787.S2CID21832575.
