Linaclotide
 | |
| Clinical data | |
|---|---|
| Trade names | Linzess |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613007 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChemCID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard(EPA) | |
| ECHA InfoCard | 100.243.239 |
| Chemical and physical data | |
| Formula | C59H79N15O21S6 |
| Molar mass | 1526.73g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Linaclotide,(sold under the brand nameLinzessin the US and Mexico, and asConstellaelsewhere)[6]is a drug used to treatirritable bowel syndromewithconstipationandchronic constipation with no known cause.[4][3]It has ablack box warningabout the risk of seriousdehydrationin children in the US; the most common adverse effects in others include diarrhea.[4]
It is anoligopeptideagonistofguanylate cyclase 2Cand remains in theGI tractafter it is takenby mouth.It was approved in the US and the European Union in 2012.[7]
It is marketed byAbbvie(formerlyAllergan) in the United states and byAstellasin Asia;[citation needed]Ironwood Pharmaceuticalswas the originator.[8][failed verification]In 2021, it was the 246th most commonly prescribed medication in the United States, with more than 1million prescriptions.[9][10]
Medical use[edit]
Linaclotide isindicatedto treatirritable bowel syndromewith constipation and chronic constipation with no known cause.[4][3]
In June 2023, the indication was expanded in the US to include the treatment offunctional constipation.[4][11]
Adverse effects[edit]
The US label has a black box warning to not use linaclotide in children less than six years old and to avoid in people from 6 to 18 years old, due to the risk of serious dehydration.[4]
More than 10% of people taking linaclotide have diarrhea. Between 1% and 10% of people have decreased appetite, dehydration, low potassium,dizziness when standing up too quickly,nausea, vomiting, urgent need to defecate, fecal incontinence, and bleeding in the colon, rectum, and anus.[3]
It has not been tested in pregnant women and it is unknown if it is excreted in breast milk.[3]
Pharmacology[edit]
Systemic absorption of the globular tetradecapeptide is minimal.[12][13]
Linaclotide, like theendogenousguanylinanduroguanylinit mimics, is anagonistthat activates thecell surface receptorofguanylate cyclase 2C(GC-C).[12][4][14]The medication binds to the surface of theintestinalepithelialcells.[4]Linaclotide is minimally absorbed and it is undetectable in the systemic circulation at therapeutic doses.[12]Activation of GC-C increasescyclic guanosine monophosphate(cGMP).[4]Elevated cGMP stimulates secretion of chloride and bicarbonate and water into the intestinal lumen, mainly by way ofcystic fibrosis transmembrane conductance regulator(CFTR)ion channelactivation.[4][15]This results in increased intestinal fluid and accelerated transit.[4]
Chemistry[edit]
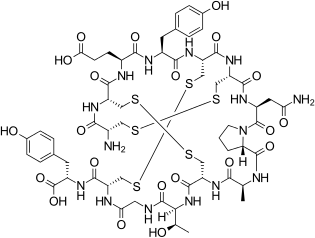
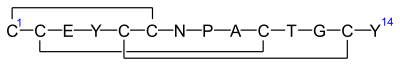
Linaclotide is a hybridpeptidedesign of theE.coliheat-stableenterotoxin(STa) and the endogenous peptide hormonesendogenousguanylinanduroguanylin.[16][12][14]It is asynthetictetradecapeptide (14amino acidpeptide) with the sequence CCEYCCNPACTGCY by one-letter abbreviation,[citation needed]or by three-letter abbreviation:[17]
H–Cys1–Cys2–Glu3–Tyr4–Cys5–Cys6–Asn7–Pro8–Ala9–Cys10–Thr11–Gly12–Cys13–Tyr14–OH
However, the actual structure of linaclotide is not fully specified without the threedisulfide(R-S-S-R) bonds it contains, which are between Cys1and Cys6,between Cys2and Cys10,and between Cys5and Cys13;[17]these are shown in exaggerated fashion in the line-angle graphic showing the chemical bonds within and between each amino acid (and their stereochemistries, see the infobox, above right), and are represented using a one-letter abbreviations in the following additional schematic:[citation needed]
A study in discovery synthesis reported that 2 of 14 strategies available to synthesize linaclotide were successful—the successful ones involvingtritylprotectionof all cysteines, or trityl protection of all cysteines except Cys1and Cys6,which were protected withtert-butylsulphenyl groups.The study also reported that solution-phase oxidation (disulfide formation) was advisable over solid-supported synthesis for linaclotide, and that the Cys1–Cys6disulfide bridge was the most favored energetically.[17]
History[edit]
The drug was discovered atMicrobia, Inc,which had been spun out of theWhitehead Institutein 1998 by postdocs from the lab ofGerald Finkto commercialize the lab's know-how and inventions related to microbial pathogens and biology.[18][19]In 2002 the company hired Mark Currie who had worked at theSearledivision ofMonsantoand then had gone toSepracor.[18]Currie directed the efforts that led to the discovery of linaclotide, which was based on anenterotoxinproduced by some strains ofEscherichia colithat causetraveler's diarrhea.[20][21]The company started Phase I trials in 2004.[18]
Under a partnership agreement announced in 2007, betweenForest Laboratoriesand Microbia, Forest would pay $70 million in licensing fees towards the development of linaclotide, with profits shared between the two companies in the US; Forest obtained exclusive rights to market in Canada and Mexico.[22]By 2010, Microbia had changed its name to Ironwood Pharmaceuticals and had licensed rights to distribute the drug in Europe toAlmiralland had licensed Asian rights toAstellas Pharma.[23]
It was approved in the United States and in the European Union in 2012.[7]
Forest was acquired in 2014 and eventually became part ofAllergan.[24]Allergan acquired rights from Almirall in 2015,[25]and in 2017, acquired remaining rights in most of the rest of the world, excluding North America, Japan, and China.[26]
Society and culture[edit]
Economics[edit]
In 2014, Ironwood and Forest then Allergan began runningdirect-to-consumer advertisingwhich raised sales by 21%; campaigns in 2015 and 2016 raised sales by 27% and 30%.[27]
In 2017, the list price for linaclotide in the US wasUS$378for 30 pills; Allergan and Ironwood increased the price of linaclotide to around $414 in 2018.[8]
References[edit]
- ^Oh SA (17 August 2011)."Macrocycle Milestone for Ironwood Pharma".The Haystack.Archived fromthe originalon 27 November 2018.Retrieved11 February2017– via CENBlog.org.
- ^"FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)".nctr-crs.fda.gov.FDA.Retrieved22 October2023.
- ^abcde"UK label: Linaclotide Summary of Product Characteristics".Electronic Medicines Compendium. September 2017.Archivedfrom the original on 15 April 2018.Retrieved15 April2018.
- ^abcdefghijk"Linzess- linaclotide capsule, gelatin coated".DailyMed.31 August 2021.Archivedfrom the original on 29 March 2021.Retrieved12 June2023.
- ^"Constella EPAR".European Medicines Agency.24 May 2023.Archivedfrom the original on 22 June 2021.Retrieved12 June2023.
- ^"Linaclotide - Ironwood Pharmaceuticals".AdisInsight.Archivedfrom the original on 7 October 2017.Retrieved15 April2018.
- ^abYu SW, Rao SS (September 2014)."Advances in the management of constipation-predominant irritable bowel syndrome: the role of linaclotide".Therapeutic Advances in Gastroenterology.7(5): 193–205.doi:10.1177/1756283X14537882.PMC4107700.PMID25177366.
- ^abNocera J (9 January 2018)."How Allergan Continues to Make Drug Prices Insane".Bloomberg News.Archivedfrom the original on 15 April 2018.Retrieved15 April2018.
- ^"The Top 300 of 2021".ClinCalc.Archivedfrom the original on 15 January 2024.Retrieved14 January2024.
- ^"Linaclotide - Drug Usage Statistics".ClinCalc.Retrieved14 January2024.
- ^"FDA approves first treatment for pediatric functional constipation".U.S.Food and Drug Administration(FDA)(Press release). 12 June 2023.Retrieved12 June2023.
- ^abcdHussain ZH, Everhart K, Lacy BE (February 2015)."Treatment of Chronic Constipation: Prescription Medications and Surgical Therapies".Gastroenterology & Hepatology.11(2): 104–114.PMC4836568.PMID27099579.
- ^Corsetti M, Tack J (February 2013)."Linaclotide: A new drug for the treatment of chronic constipation and irritable bowel syndrome with constipation".United European Gastroenterology Journal.1(1): 7–20.doi:10.1177/2050640612474446.PMC4040778.PMID24917937.
- ^abLove BL, Johnson A, Smith LS (July 2014). "Linaclotide: a novel agent for chronic constipation and irritable bowel syndrome".American Journal of Health-System Pharmacy.71(13): 1081–1091.doi:10.2146/ajhp130575.PMID24939497.
- ^Yu SW, Rao SS (September 2014)."Advances in the management of constipation-predominant irritable bowel syndrome: the role of linaclotide".Therapeutic Advances in Gastroenterology.7(5): 193–205.doi:10.1177/1756283X14537882.PMC4107700.PMID25177366.
- ^Braga Emidio N, Tran HN, Andersson A, Dawson PE, Albericio F, Vetter I, Muttenthaler M (June 2021)."Improving the Gastrointestinal Stability of Linaclotide".Journal of Medicinal Chemistry.64(12): 8384–8390.doi:10.1021/acs.jmedchem.1c00380.PMC8237258.PMID33979161.
- ^abcGóngora-Benítez M, Tulla-Puche J, Paradís-Bas M, Werbitzky O, Giraud M, Albericio F (2011)."Optimized Fmoc solid-phase synthesis of the cysteine-rich peptide linaclotide"(PDF).Biopolymers.96(1): 69–80.doi:10.1002/bip.21480.PMID20560145.S2CID46150263.Archived fromthe original(PDF)on 11 February 2017.Retrieved10 February2017.
- ^abcWithers M (22 September 2004)."Druhunters".Paradigm Magazine, Whitehead Institute.Archivedfrom the original on 28 August 2021.Retrieved15 April2018.
- ^Timmerman L (23 February 2009)."Xconomy: Renewables Aren't Just for Biofuels: Microbia Makes Industrial Chemicals a Bit Greener".Xconomy.Archivedfrom the original on 16 April 2018.Retrieved15 April2018.
- ^Hornby PJ (2015). "Drug discovery approaches to irritable bowel syndrome".Expert Opinion on Drug Discovery.10(8): 809–824.doi:10.1517/17460441.2015.1049528.PMID26193876.S2CID207494271.
- ^"Director profile: Mark Currie, Ph.D."MUSC Foundation for Research Development. Archived fromthe originalon 16 April 2018.Retrieved15 April2018.
- ^"Microbia, Forest Laboratories Announce Linaclotide Collaboration".FDA News.17 September 2007.Archivedfrom the original on 20 August 2016.Retrieved15 September2010.
- ^Pollack, Andrew (13 September 2010)."Drug for Irritable Bowel Achieves Goals in Trial".The New York Times.Archivedfrom the original on 20 December 2011.Retrieved14 September2010.
- ^Jones S, Burdette K, Wieczner J (30 July 2015)."From Actavis to Allergan: One pharma company's wild dealmaking journey".Fortune.Archivedfrom the original on 27 September 2020.Retrieved15 April2018.
- ^"Press release: Allergan Acquires Rights To Ironwoods Constella (Linaclotide) From Almirall In More Than 40 Countr".Allergan.27 October 2015.Archivedfrom the original on 15 April 2018.Retrieved15 April2018.
- ^"8-K"(PDF).Ironwood. 31 January 2017. Archived fromthe original(PDF)on 15 April 2018.Retrieved15 April2018.
- ^LaMotta L."How DTC got things moving for Linzess".BioPharma Dive.Archivedfrom the original on 15 April 2018.Retrieved15 April2018.
